Preparation method of tetraene intermediates
A technology for intermediates and tetraenes, applied in the field of preparation of tetraenes intermediates, can solve the problems of many by-products, low yield of target products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
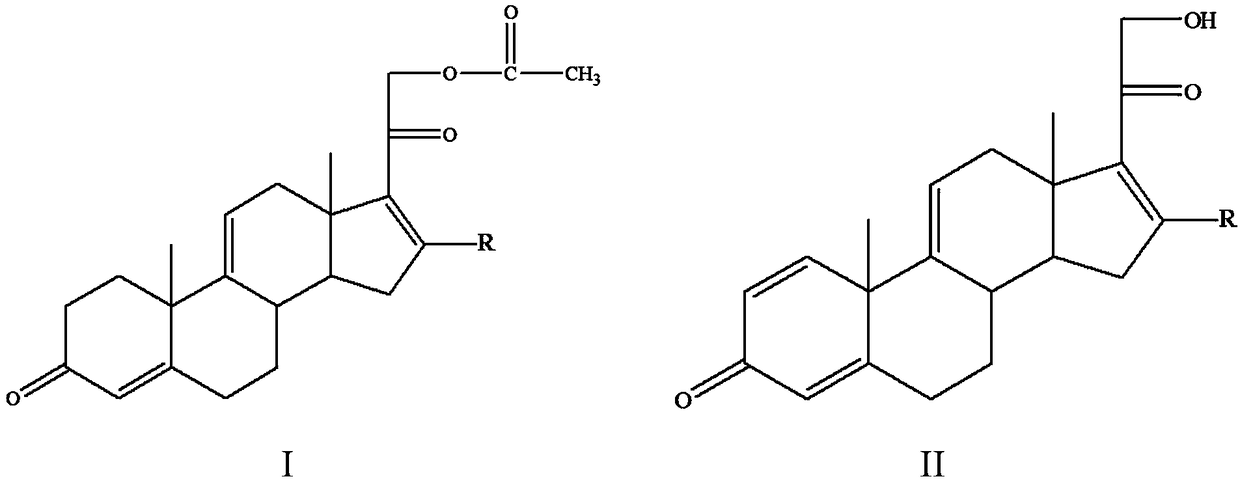
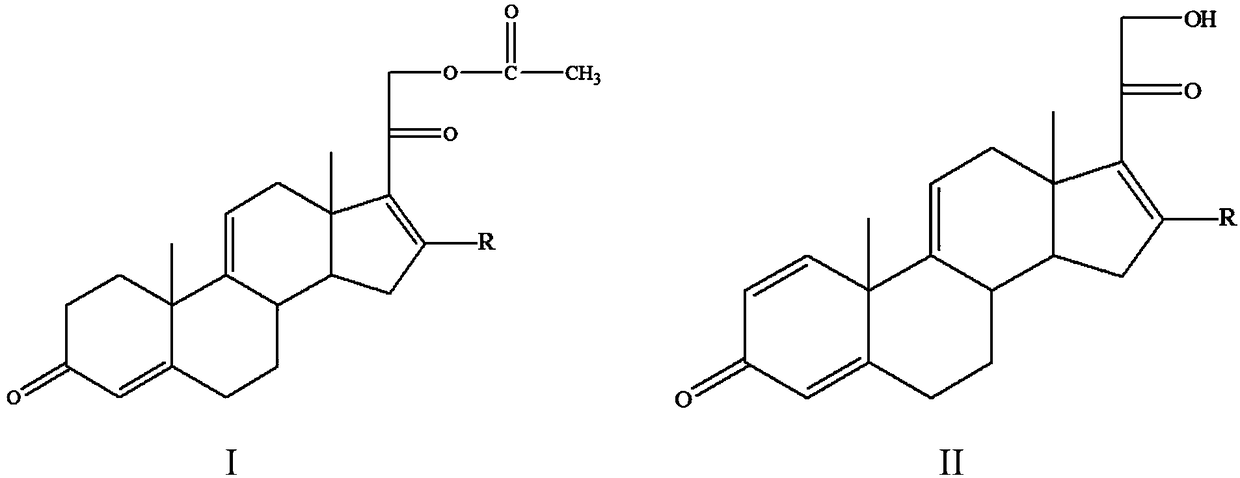
[0023] A method for preparing a tetraene intermediate according to an embodiment of the present invention includes the following steps: using Nocardia simplex to perform microbial transformation on the first compound to obtain a tetraene intermediate, the first compound is shown in general formula I, four The alkene intermediate is shown in general formula II,
[0024]
[0025] In the general formula I and the general formula II, R is H, a halogen atom, an alkyl group, an alkoxy group, a hydroxyl group or a phenyl group.
[0026] In this embodiment, the first compound represented by the above general formula I is selected as the substrate, and only Nocardioides simplex is used for biotransformation, and the substrate is simultaneously dehydrogenated at positions 1 and 2 and acetate at position 21 hydrolysis to obtain a tetraene intermediate, and the hydrolysis of the 21-position acetate can effectively promote the dehydrogenation of the 1 and 2 positions, so that most of th...
Embodiment 1
[0049] Put 6 liters of fermentation medium into 10 liters of fermenters, inoculate 100 mL of Nocardia simplex seed liquid (thalline concentration: 2.4 × 10 9 cells / ml), culture was started, and the culture conditions were temperature 31°C, rotation speed 180 rpm, and culture time 16 hours.
[0050] Samples were diluted 30 times and detected at 580 nm with a spectrophotometer, and the OD value was 0.58. 120g of 2TR, 4.8g of PPE and 1.2g of Tween 80 were added to the fermenter to start the fermentative conversion. The culture conditions during fermentation transformation were temperature 31±1°C, rotation speed 200rpm, air flow rate 0.2-0.3vvm, tank pressure 0.05Mpa, after 72 hours of transformation, samples were taken and sent for HPLC analysis. The results are shown in Table 1:
[0051] Table 1
[0052] name
substrate
tetraenyl acetate
2TR Deesterified
target product
purity
0.56%
6.81%
0.15%
93.25%
[0053] After the conversio...
Embodiment 2
[0067] Put 6 liters of fermentation medium into a 10 liter fermenter, inoculate 100 mL of Nocardia simplex seed liquid (thalline concentration: 3.2×10 9 cells / ml), culture was started, and the culture conditions were temperature 31°C, rotation speed 180 rpm, and culture time 16 hours.
[0068] Samples were diluted 30 times and detected at 580 nm with a spectrophotometer, and the OD value was 0.45. 180g of 2TR, 4.8g of PPE and 1.2g of Tween 80 were added to the fermenter to start the fermentative conversion. The culture conditions during fermentation transformation were temperature 31±1°C, rotation speed 200rpm, air flow rate 0.2vvm, tank pressure 0.05Mpa, after 72 hours of transformation, samples were taken and sent for HPLC analysis. The results are shown in Table 5:
[0069] table 5
[0070] name
substrate
tetraenyl acetate
2TR Deesterified
target product
purity
0.66%
4.62%
0.45%
94.15%
[0071] After the conversion is comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com