A kind of hindered amine modified lignin and its preparation method and application
A technology of lignin and hindered amine, which is applied in the field of lignin materials, can solve the problems that the UV protection performance is difficult to achieve chemically synthesized light stabilizers, serious molecular agglomeration, and low content of functional groups, etc., to achieve rich types and application ranges, and simple production process , the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Dissolve 30g of 2,2,6,6-tetramethyl-4-piperidinol and 10g of epichlorohydrin in 20g of dimethylformamide, heat to 80°C, melt and mix evenly, add 1g of three A boron fluoride catalyst was reacted at 80° C. for 2 hours to obtain a chlorotetramethylpiperidine intermediate;
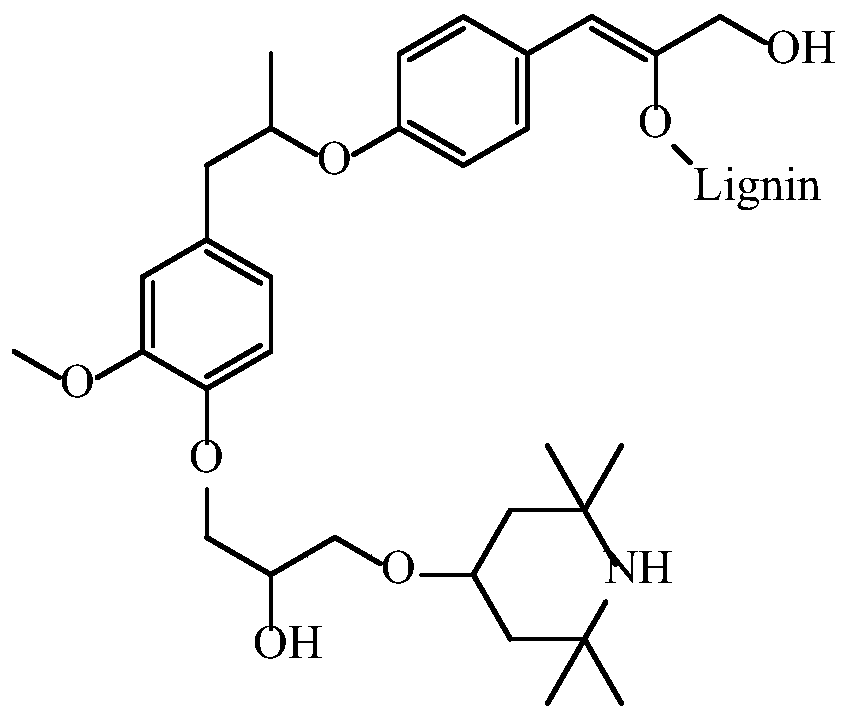
[0036] (2) Dissolve 100g of lignosulfonate in alkaline aqueous solution, prepare 20% solid content, adjust pH=10; then heat up to 90°C, add dropwise the chlorotetramethylpiperene prepared in step (1) Pyridine intermediate, after dripping, keep warm at 90°C for 1 hour to obtain tetramethylpiperidine grafted modified lignin, the structure diagram is shown in figure 1 .
Embodiment 2
[0038] (1) Dissolve 50g of 2,2,6,6-tetramethyl-4-piperidinol and 20g of epichlorohydrin in 40g of dimethylacetamide, heat to 50°C, melt and mix evenly, add 1g of chloride Aluminum catalyst, reacted for 1.5 hours at 50°C to obtain a chlorotetramethylpiperidine intermediate;
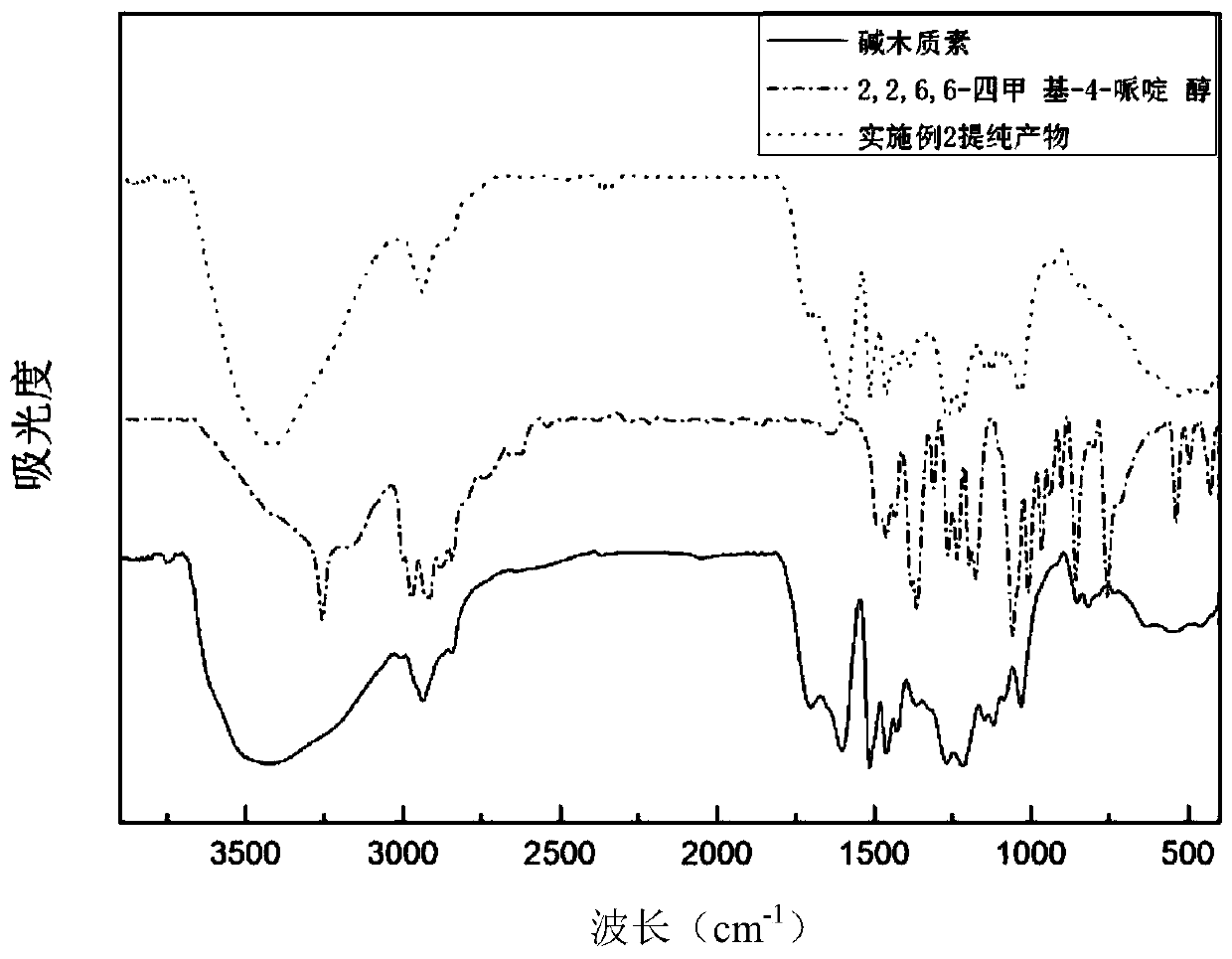
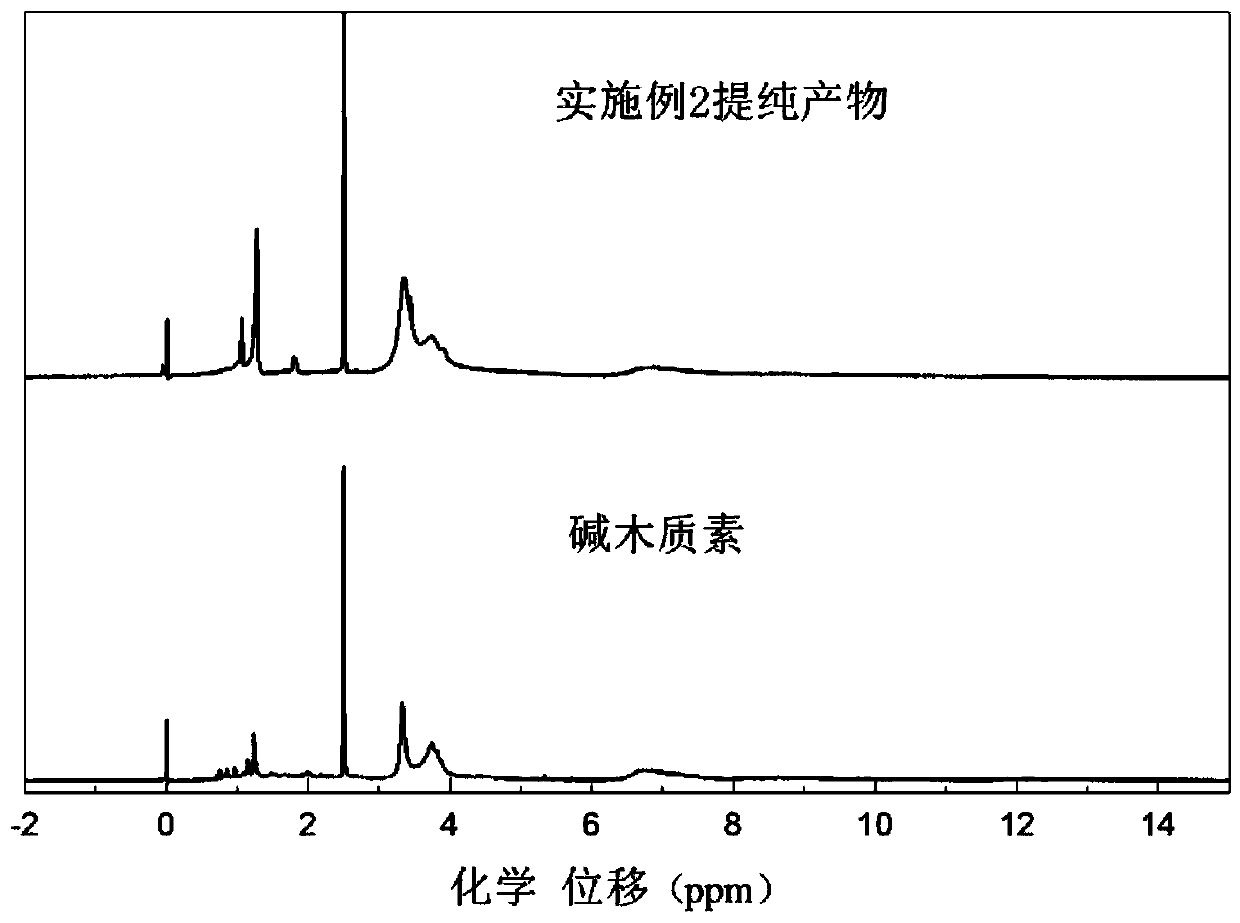
[0039] (2) Dissolve 100g of alkali lignin in an alkaline aqueous solution to prepare a solid content of 25%, and adjust the pH to 12; then heat up to 80°C, and add dropwise the chlorinated tetramethylpiperidine prepared in step (1) After the dripping is completed, keep the reaction at 80°C for 2 hours to obtain tetramethylpiperidine grafted modified lignin.
Embodiment 3
[0041] (1) Dissolve 60g of 2,2,6,6-tetramethyl-4-piperidinol and 20g of epichlorohydrin in 20g of tetrahydrofuran and 20g of dioxane, heat to 50°C, melt and mix well, add 1.5g Tin tetrachloride catalyst, heating up at 55 DEG C and reacting for 2 hours, obtains chlorotetramethylpiperidine intermediate;
[0042] (2) Dissolve 100g of solvent-based lignin in an alkaline aqueous solution to prepare a solid content of 40%, and adjust the pH to 14; then heat up to 70°C, and add the chlorotetramethylpiperidine prepared in step (1) dropwise The intermediate, after the dripping is completed, is kept at 70° C. for 3 hours and reacted to obtain tetramethylpiperidine grafted modified lignin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com