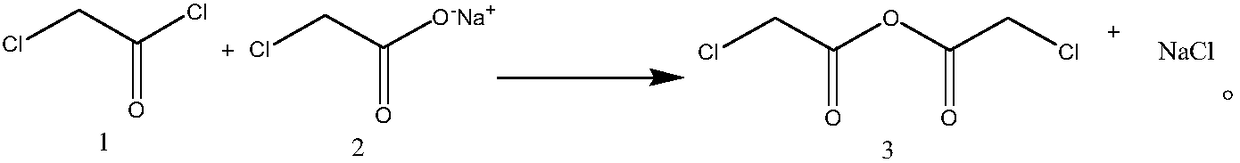
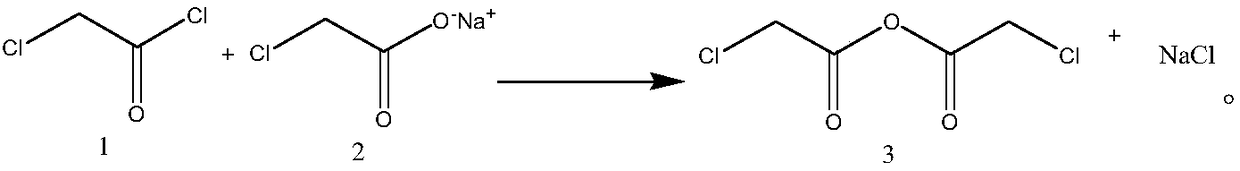
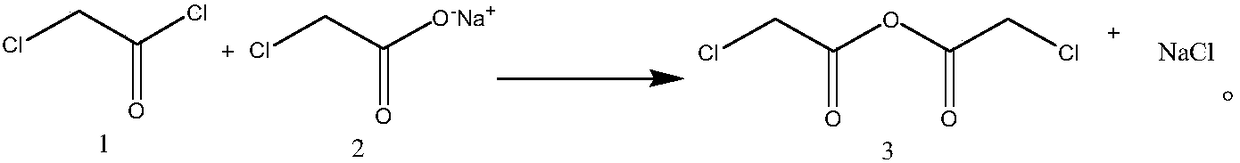
Synthesis process of chloroacetic anhydride
A technology for the synthesis of chloroacetic anhydride, which is applied in the field of synthesis of chloroacetic anhydride, can solve the problems of equipment corrosion, strong corrosion of equipment, and high requirements for equipment, and achieve the goals of less equipment investment, good product quality, and reduced process energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: a kind of synthetic technique of chloroacetic anhydride, comprises the following steps:
[0027] Step 1, preparation of chloroacetic anhydride solution: add 1 equivalent of sodium chloroacetate to the reaction flask, then add an aprotic solvent, the weight of the aprotic solvent is twice that of sodium chloroacetate, heat to 40°C, stir, and slowly drop 0.9 equivalents of chloroacetyl chloride, after 2 hours of dropwise addition, continue to stir at 40°C for 1 hour, filter, filter the filter cake after washing with an aprotic solvent, and combine the filtrates to obtain a chloroacetic anhydride solution with a purity of 95%. The rate is 95%, wherein the aprotic solvent is toluene;
[0028] Step 2, purification of chloroacetic anhydride: add the above-mentioned chloroacetic anhydride solution into the reactor, add petroleum ether, cool to 35°C, continue stirring until the solid precipitates, cool down to 0°C, keep stirring for 1 hour, filter, and use for fil...
Embodiment 2
[0031] Embodiment 2: a kind of synthetic technique of chloroacetic anhydride, the difference with embodiment one is, comprise the following steps: step 1, the preparation of chloroacetic anhydride solution: add 1 equivalent sodium chloroacetate in reaction bottle, then add Aprotic solvent, the weight of the aprotic solvent is 3 times that of sodium chloroacetate, heated to 40 ° C, stirred, slowly added dropwise 1 equivalent of chloroacetyl chloride, after 2 hours, the addition was completed, and continued to stir at 40 ° C for 1 hour, Filtration, the filter cake was washed with an aprotic solvent and filtered, and the filtrates were combined to obtain a chloroacetic anhydride solution with a purity of 95% and a yield of 95.1%, wherein the aprotic solvent was toluene;
[0032] Step 2, purification of chloroacetic anhydride: add the above-mentioned chloroacetic anhydride solution into the reactor, add petroleum ether, cool to 35°C, continue stirring until the solid precipitates, ...
Embodiment 3
[0033] Embodiment 3: a kind of synthetic technique of chloroacetic anhydride, differs from embodiment one in, comprises the following steps: Step 1, the preparation of chloroacetic anhydride solution: add 1 equivalent sodium chloroacetate in reaction bottle, then add Aprotic solvent, the weight of the aprotic solvent is 5 times that of sodium chloroacetate, heated to 40 ° C, stirred, slowly added dropwise 1.2 equivalents of chloroacetyl chloride, after 2 hours, the addition was completed, and continued to stir at 40 ° C for 1 hour, Filtration, the filter cake was washed with an aprotic solvent and filtered, and the filtrates were combined to obtain a chloroacetic anhydride solution with a purity of 95.1% and a yield of 95.3%, wherein the aprotic solvent was toluene;
[0034] Step 2, purification of chloroacetic anhydride: add the above-mentioned chloroacetic anhydride solution into the reactor, add petroleum ether, cool to 35°C, continue stirring until the solid precipitates, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com