Gliquidone crystal, preparation method thereof and drug containing gliquidone crystal
A technology for gliquidone and crystals, applied in the field of medicinal chemistry, can solve the problems of low purity, poor stability and low safety of gliquizone, and achieve high product yield, high stability and good repeatability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The method for the preparation of gliquidone crystals adopted in the present embodiment is as follows:
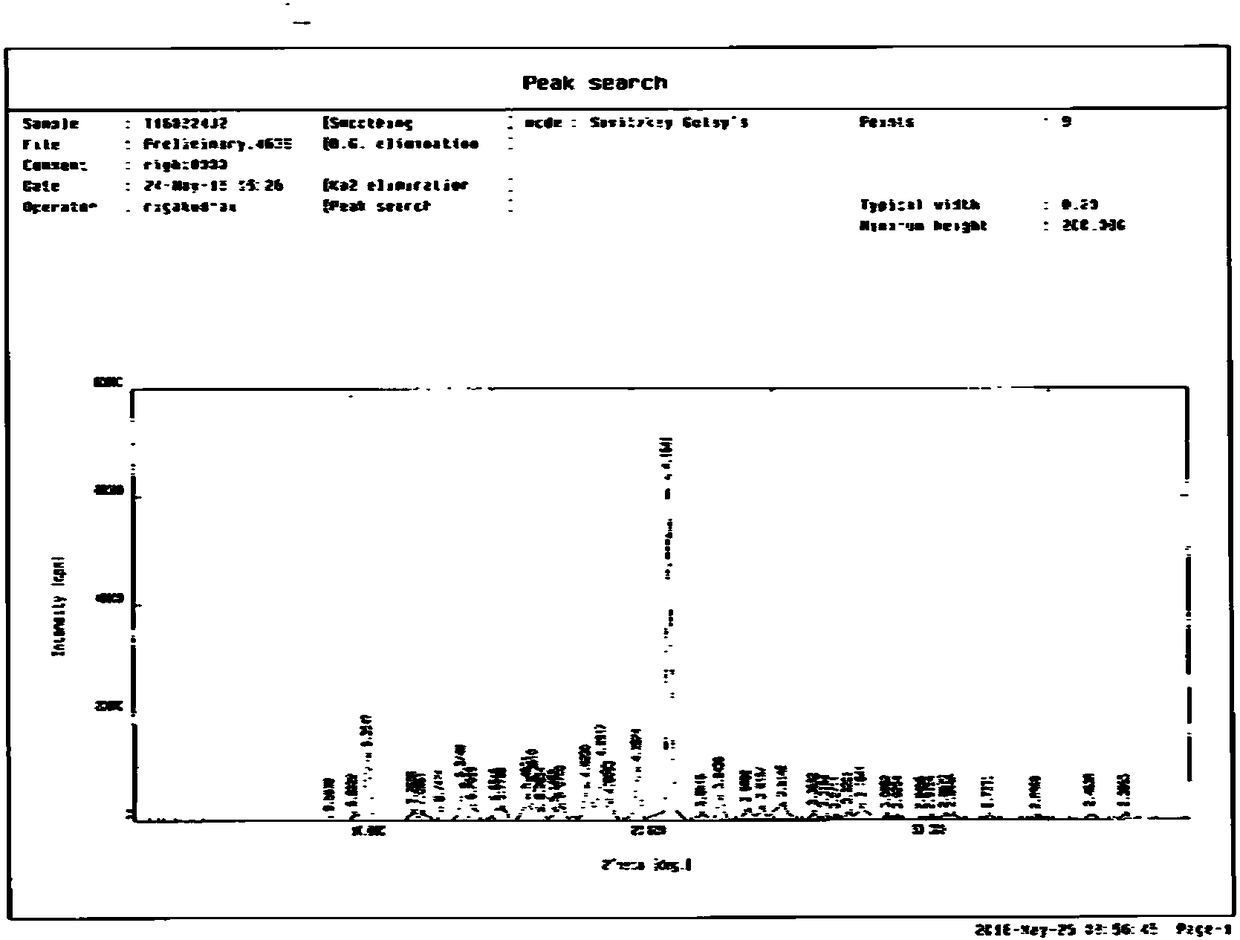
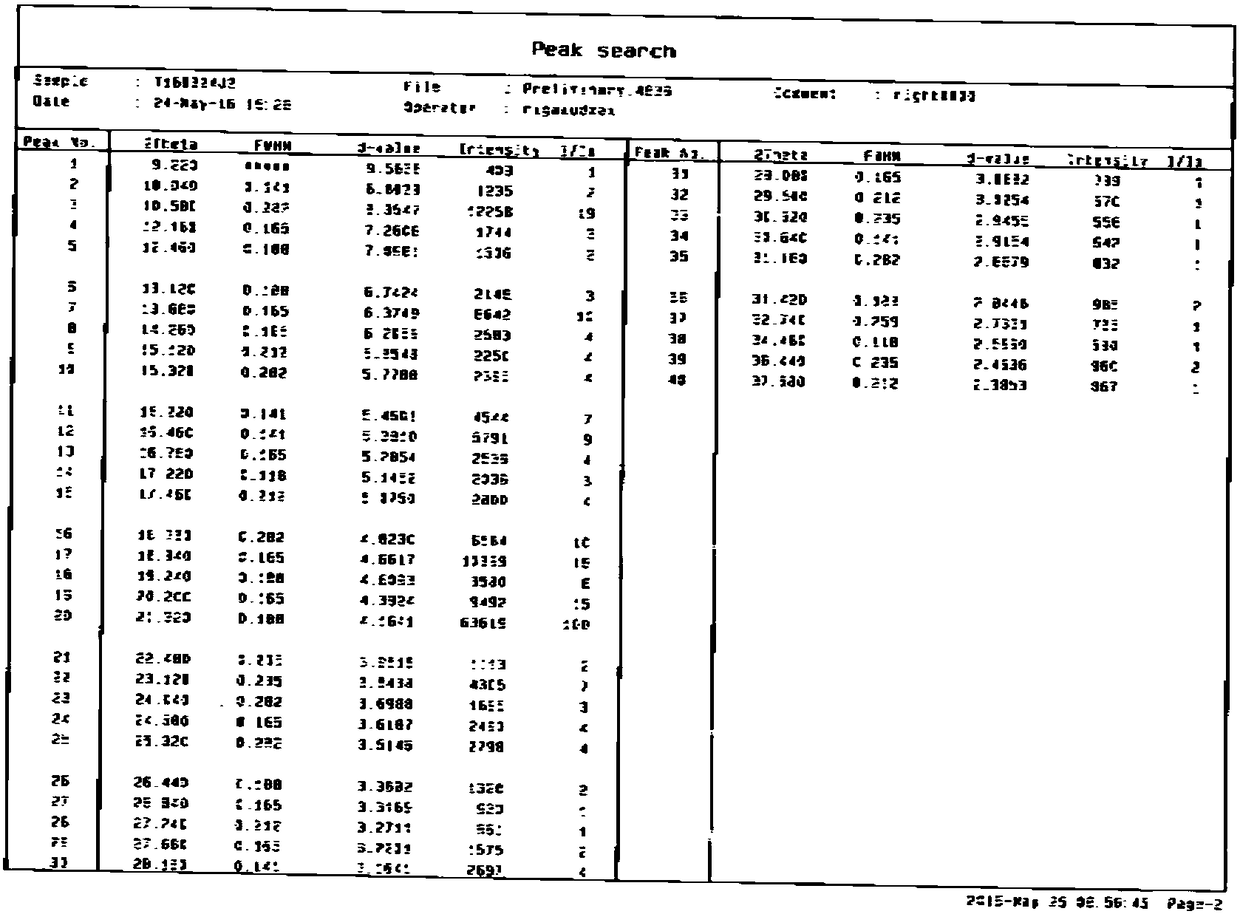
[0033] Take 10 g of crude gliquidone and put it into a three-necked flask, add 20 g of acetonitrile, start stirring and heating. Heat it to reflux, and keep it under reflux, slowly add acetonitrile dropwise until it is completely dissolved (approximately 23g can be added to dissolve completely), after the dissolution is complete, continue to reflux for 2 hours, then filter while it is hot, and lower the temperature to 0-5°C with ice-salt water , and then heat and crystallize for 4 hours, filter, and dry the obtained solid in a blast oven at 50-60° C. for 6-8 hours to obtain 9.1 g of off-white solid with a yield of 91%. The purity of the gliquidone crystal form is 98.5%. , isoquinolines not exceeding 0.6%, figure 1 for its XRPD pattern, figure 2 For its XRPD data, it can be seen from the XRPD pattern and data results that the obtained gliquidone crystal form is A c...
Embodiment 2
[0035] The method for the preparation of gliquidone crystals adopted in the present embodiment is as follows:
[0036] Take 10 g of gliquidone and add it into a three-necked flask, add 20 g of acetonitrile, start stirring and heating. Heat it to reflux, and keep it under reflux, slowly add acetonitrile dropwise until it is completely dissolved (approximately 23g can be added to completely dissolve), after the dissolution is complete, continue to reflux for 2 hours, then filter while it is hot, and lower the temperature of ice-salt water to 0-5°C , and then heat and crystallize for 6 hours, filter, and dry the obtained solid in a blast oven at 50-60° C. for 6-8 hours. 9.0 g of off-white solid was obtained, the yield was 90%, the crystal form of gliquidone had a purity of 98.3%, and the isoquinoline was no more than 0.6%. figure 1 for its XRPD pattern, figure 2 For its XRPD data, it can be seen from the XRPD pattern and data results that the obtained gliquidone crystal form i...
Embodiment 3
[0038] The method for the preparation of gliquidone crystals adopted in the present embodiment is as follows:
[0039] Take 10 g of gliquidone and add it into a three-necked flask, add 20 g of acetonitrile, start stirring and heating. Heat it to reflux, and keep it under reflux, slowly add acetonitrile dropwise until it is completely dissolved (approximately 23g can be added to completely dissolve), after the dissolution is complete, continue to reflux for 2 hours, then filter while it is hot, and lower the temperature of ice-salt water to 0-5°C , and then heat and crystallize for 4 hours, filter, and dry the obtained solid in a blast oven at 60-70° C. for 6-8 hours. 9.1 g of off-white solid was obtained with a yield of 91%, the purity of the gliquidone crystal form was 98.2%, and the isoquinoline was no more than 0.6%. figure 1 for its XRPD pattern, figure 2 For its XRPD data, it can be seen from the XRPD pattern and data results that the obtained gliquidone crystal form i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com