Chemical copper plating solution and chemical copper plating method
A technology of electroless copper plating and cuprous chloride, which is applied in the field of electroless plating, can solve the problems of unstable plating quality during electroless copper plating, decreased production efficiency, and low copper plating speed, so as to ensure quality, improve plating speed, good stabilizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
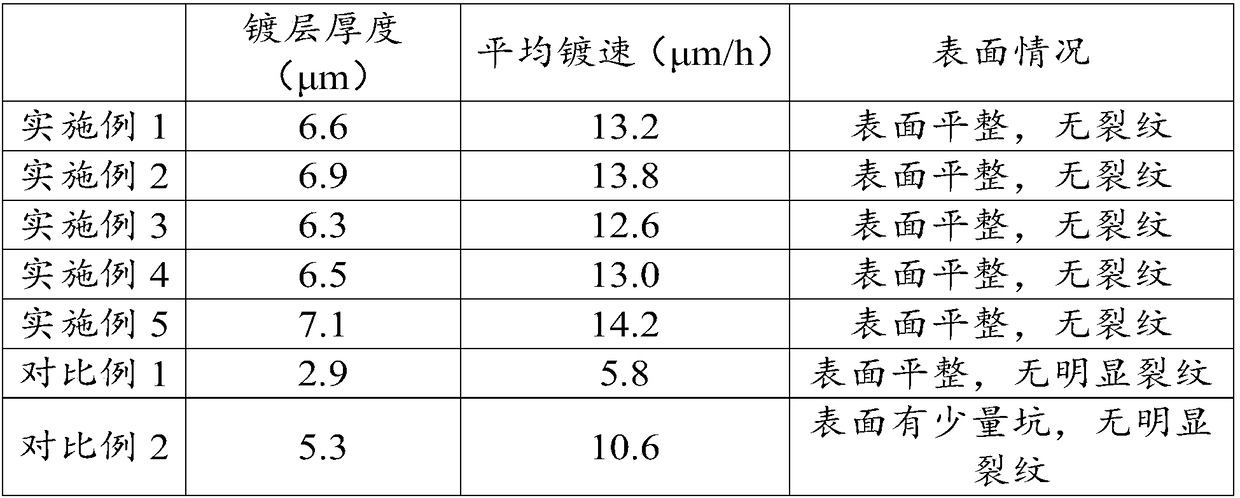
Examples
Embodiment 1
[0036] The present embodiment provides a kind of electroless copper plating solution, it comprises:
[0037] Cuprous salt (cuprous chloride) 10g / L, formaldehyde 7.5g / L, phosphine ligand (triphenylphosphine) 25g / L, auxiliary complexing agent (ethylenediamine) 0.5g / L, stabilizer (linked pyridine) 1g / L, surfactant (sodium dodecylbenzenesulfonate) 0.5g / L.
[0038] Its preparation method is as follows:
[0039] S1. Dissolving the phosphine ligand, auxiliary complexing agent, and surfactant in deionized water, and stirring evenly to obtain the first solution;
[0040] S2. Add a stabilizer and formaldehyde to the first solution, stir evenly, and adjust the pH to 11 with sodium hydroxide to obtain a second solution;
[0041] S3. Add cuprous salt to the above second solution, stir until dissolved, and age at room temperature for 24 hours to obtain the desired electroless copper plating solution.
Embodiment 2
[0043] The present embodiment provides a kind of electroless copper plating solution, it comprises:
[0044] Cuprous salt (cuprous chloride) 20g / L, formaldehyde 15g / L, phosphine ligand (triphenylphosphine) 50g / L, auxiliary complexing agent (sodium citrate) 2.5g / L, stabilizer (bipyridyl ) 1g / L, surfactant (sodium dodecylbenzenesulfonate) 0.1g / L.
[0045] Its preparation method is as follows:
[0046] S1. Dissolving the phosphine ligand, auxiliary complexing agent, and surfactant in deionized water, and stirring evenly to obtain the first solution;
[0047] S2. Add a stabilizer and formaldehyde to the first solution, stir evenly, and adjust the pH to 13 with sodium hydroxide to obtain a second solution;
[0048] S3. Add cuprous salt to the above second solution, stir until dissolved, and age at room temperature for 36 hours to obtain the desired electroless copper plating solution.
Embodiment 3
[0050] The present embodiment provides a kind of electroless copper plating solution, it comprises:
[0051] Cuprous salt (cuprous bromide) 15g / L, formaldehyde 10g / L, phosphine ligand (tributylphosphine) 40g / L, auxiliary complexing agent (sodium citrate) 2g / L, stabilizer (methanol) 0.5 g / L, surfactant (sodium lauryl sulfate) 0.2g / L.
[0052] Its preparation method is as follows:
[0053] S1. Dissolving the phosphine ligand, auxiliary complexing agent, and surfactant in deionized water, and stirring evenly to obtain the first solution;
[0054] S2. Add a stabilizer and formaldehyde to the first solution, stir evenly, and adjust the pH to 11 with sodium hydroxide to obtain a second solution;
[0055] S3. Add cuprous salt to the above second solution, stir until dissolved, and age at room temperature for 24 hours to obtain the desired electroless copper plating solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com