A kind of small interfering nucleic acid pharmaceutical composition and use thereof
A small interfering nucleic acid and composition technology, applied in the field of biomedicine, can solve the problems of reducing the level of HBV DNA, reducing the effect of reducing surface antigens, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
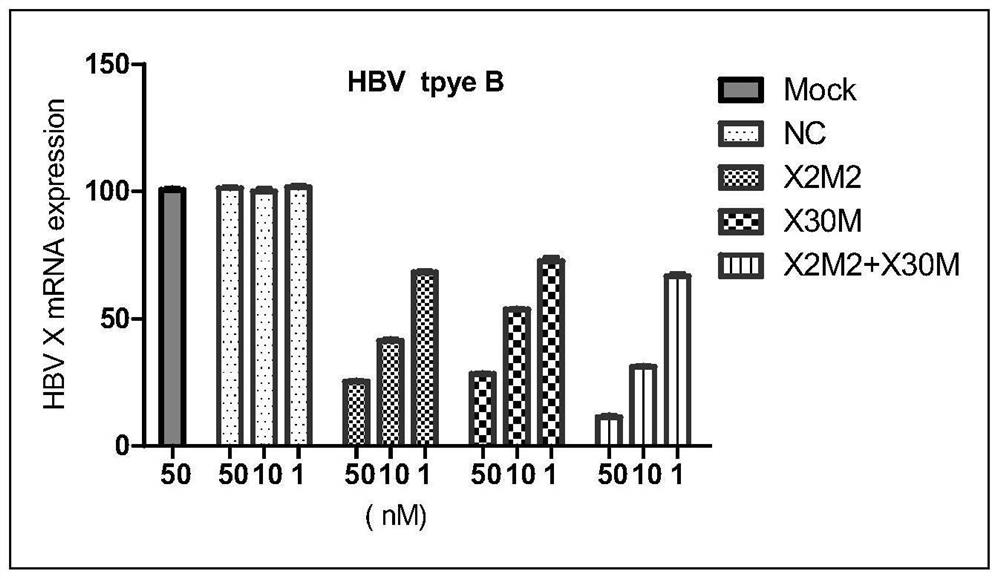
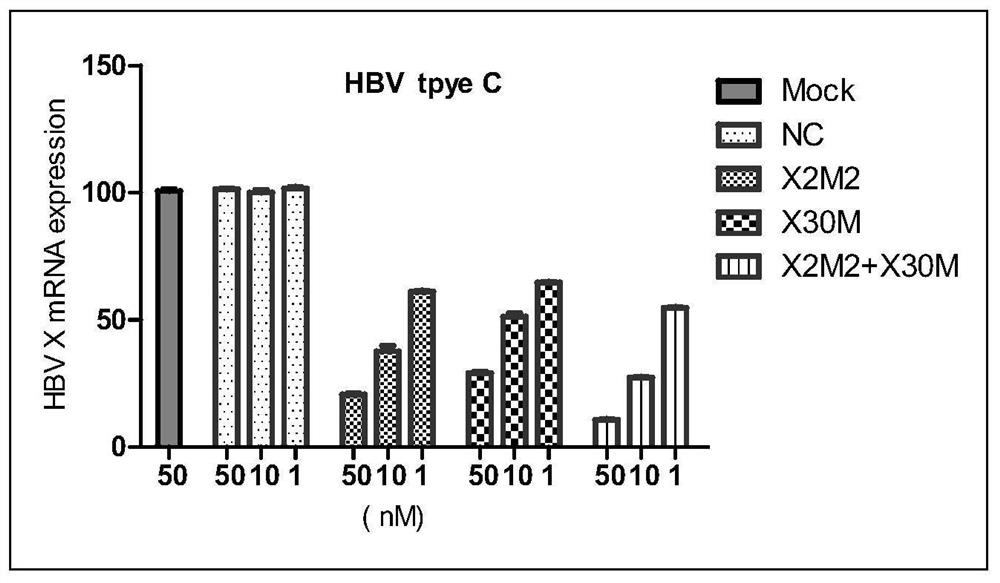
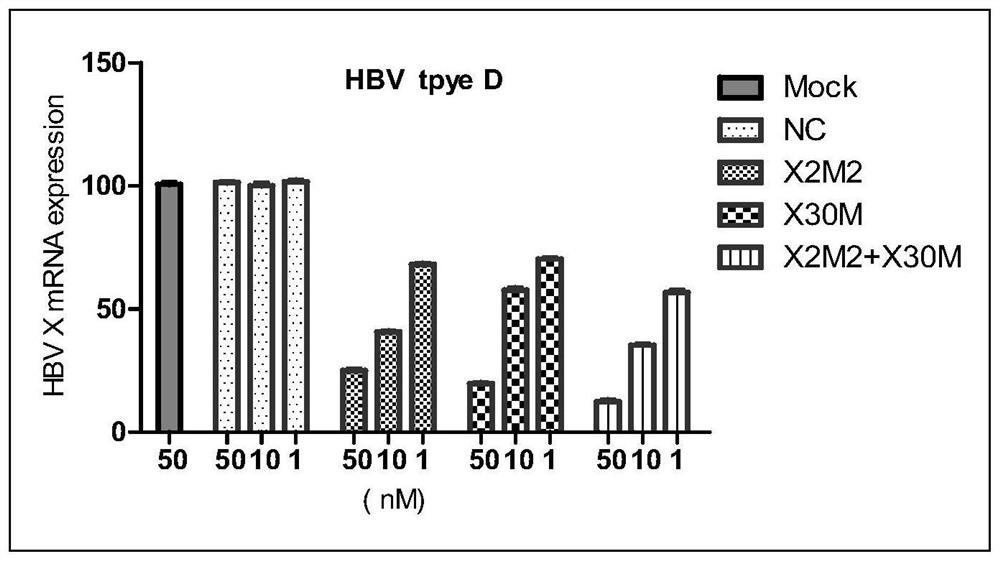
[0100] The present invention is further described in detail by the following examples, but the present invention is not limited to these examples. It should be noted that, in the following examples, although only the modified siRNA X2M2 and siRNA X30M are used to illustrate the present invention, the pharmaceutical compositions containing unmodified siRNA X2M2 and siRNA X30M also have activity and certain stability sex.
[0101] Unless otherwise specified, the reagents and culture medium used in the following examples are all commercially available products, and operations such as nucleic acid electrophoresis and real-time PCR are performed according to conventional protocols. For example, it can be carried out as described in Molecular Cloning (Cold Spring Harbor Laboratory Press (1989)).
preparation Embodiment 1
[0103] The siRNAs listed in Table 1 were obtained by conventional solid phase synthesis methods. Equimolar mixtures of sense and antisense strands were dissolved in annealing salt solution, followed by conventional annealing to form siRNA duplexes. Among them, NC is an irrelevant sequence that has no targeting effect on HBV mRNA, and as a negative control, it is also referred to as a control siRNA hereinafter; the other siRNAs (siRNA X2M2 and siRNAX30M) are siRNAs that specifically target HBV, and are also referred to below as siRNAs. called the test siRNA.
[0104] Table 1 Sequences of siRNA
[0105]
[0106] Note: Capital letters C, G, U, A and T indicate the base composition of nucleotides; lowercase letter d indicates that a nucleotide to the right of the letter d is a deoxyribonucleotide; lowercase letter m indicates the letter m The ribosyl group of a nucleotide on the left is a 2'-methoxy ribosyl group formed by replacing a 2'-hydroxyl group with a methoxy group; t...
preparation Embodiment 2
[0108] Three dry powder lipid compounds (i.e., amine-containing compound, helper lipid, pegylated lipid) were suspended in ethanol at a molar ratio of 59:29:12 and mixed, the total mass concentration of the three lipid compounds About 8.85mg / ml. The synthesized siRNAs listed in Table 1 were dissolved in 200 mM sodium acetate (pH 5.2) solution so that the siRNA concentration was 0.2 mg / ml (for the case of using siRNA X2M2 and siRNA X30M alone, the siRNA concentration was 0.2 mg). / ml; in the case of combined application of siRNAX2M2 and siRNAX30M, the two siRNAs were mixed in equal proportions, that is, the concentration of each siRNA was 0.1 mg / ml). The resulting lipid ethanol solution and siRNA sodium acetate aqueous solution were mixed very rapidly in a 1:3 volume ratio. The specific composition of the liposome formulation obtained after mixing is described in Table 2.
[0109] Table 2 Composition of liposome preparations
[0110]
[0111] The resulting liposome formul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com