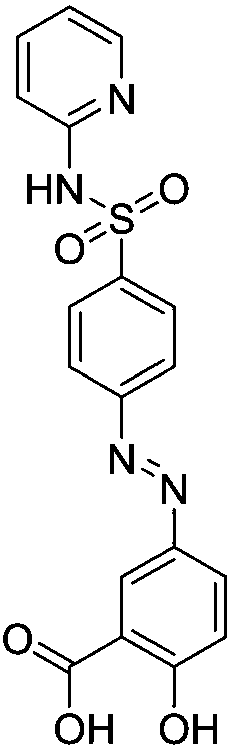
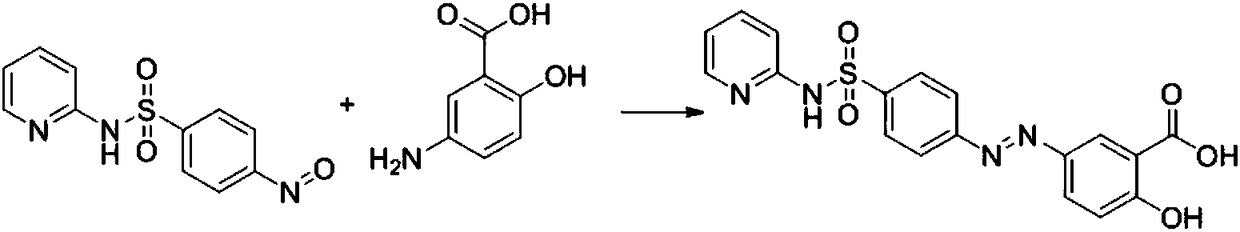
Preparation method of sulfasalazine
A technology of sulfasalazine and sulfasalazine, which is applied in the field of sulfasalazine, can solve the problems of local reaction overheating, long reaction time, and unsatisfactory product purity, and achieve the effect of less reaction steps and reagent safety and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of 4-nitrososulfopyridine:
[0031] Add sodium nitrite (1.1eq) and dilute hydrochloric acid (2.0eq) to 4-aminosulfapyridine in methanol, and heat at 60°C for 3h. After the reaction was completed, the solvent was removed, water was added, and ethyl acetate was extracted. The obtained ethyl acetate organic phase was combined, dried and spin-dried, and the obtained solid crude product was recrystallized from acetone to obtain 4-nitrososulfopyridine.
Embodiment 1
[0033] Add 2.9g of the formula 5-aminosalicylic acid into 50ml of water at 20-25°C and stir, then add hydrochloric acid to adjust the pH to 3, stir and dissolve, heat up to 30-35°C and keep warm. Then add dropwise a solution of 5 g of 4-nitrososulfopyridine in 10 ml of methanol at 30 to 35° C. After dropping, raise the temperature to 50° C. and keep it warm until the reaction is complete. After heat preservation, it was cooled to 0°C for suction filtration, and the material was dried by suction to obtain 7 g of sulfasalazine with a yield of 92.5%.
Embodiment 2
[0035] Add 1.45g of the formula 5-aminosalicylic acid into 50ml of water at 20-25°C and stir, then add hydrochloric acid to adjust the pH to 3, stir to dissolve, heat up to 30-35°C and keep warm. Then add dropwise a solution of 5 g of 4-nitrososulfopyridine in 10 ml of methanol at 30 to 35° C. After dropping, raise the temperature to 50° C. and keep it warm until the reaction is complete. After the heat preservation was completed, the mixture was cooled to 0°C for suction filtration, and the material was dried by suction to obtain 3.5 g of sulfasalazine with a yield of 46.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com