Method for preparing 1,3-propane sultone
A technology of propane sultone and propane sulfonic acid, which is applied in the field of preparing 1,3-propane sultone, can solve the problems of easy oxidation of acrolein, high cost, and high requirements for reaction equipment, and achieve low output of three wastes, Low toxicity, reduction of raw materials and operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
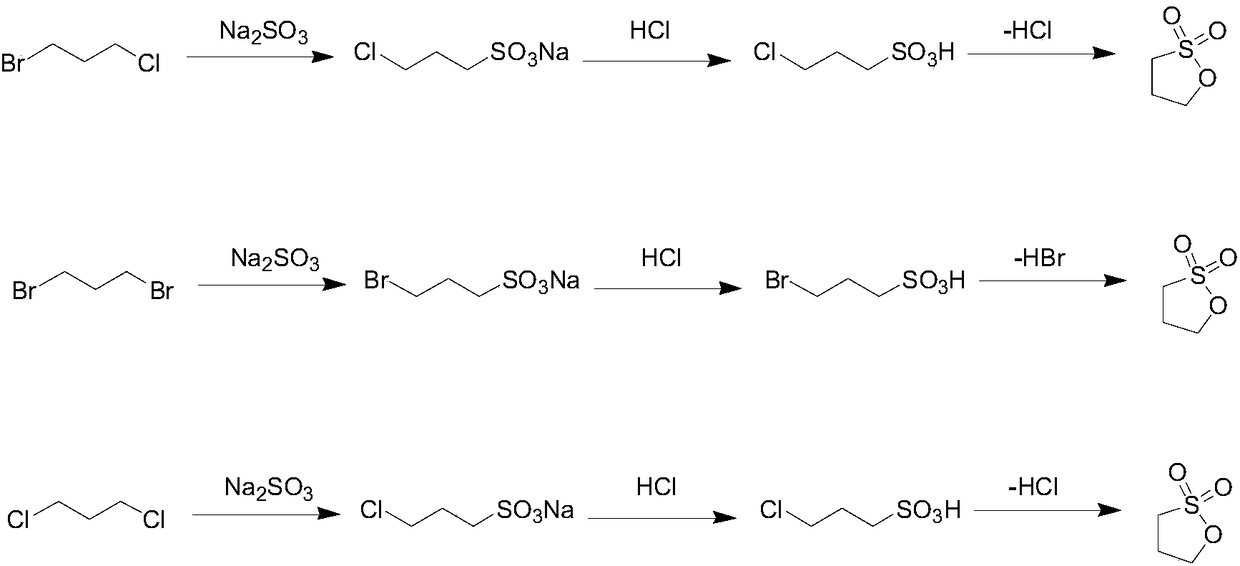
[0032] 1. Add 40g sodium sulfite (1.0eq), 50g (1.0eq) of 1-bromo-3-chloropropane, 250 milliliters of ethanol to the reaction flask, heat up to 80°C and react for 8 hours after adding, cool to 10°C, filter to obtain Crude sodium 1-chloro-3-propanesulfonate.
[0033] ②Put the crude sodium 1-chloro-3-propanesulfonate obtained in the previous step into the reaction bottle, add 100 ml of water, add hydrochloric acid below 20 degrees to adjust the pH=1-3, concentrate under reduced pressure to remove most of the water, and add 80 ml of ethanol , cooled to 10 degrees, filtered, and the filtrate was concentrated to dryness to obtain 48 g of 1-chloro-3-propanesulfonic acid.
[0034] ③ The 1-chloro-3-propanesulfonic acid obtained in the previous step was subjected to a lactonization reaction at 140-200° C. under high vacuum (-0.1 MPa), and the distillate was received at the same time to obtain 32 g of 1.3-propane sultone. The total yield of the product is 83%, and its purity is more tha...
Embodiment 2
[0036] ①Add 10.4g of sodium sulfite (1.0eq), 50g of 1,3-dibromopropane (3.0eq), and 200ml of ethanol into the reaction flask, heat up to 80°C and react for 10 hours after adding, cool down to 10°C, and filter to obtain Crude sodium 1-bromo-3-propanesulfonate, (unreacted 1,3-dibromopropane is in the filtrate and can be recovered).
[0037] ②Put the sodium 1-bromo-3-propanesulfonate obtained in the previous step into the reaction flask, add 60 ml of water, add hydrochloric acid below 20 degrees to adjust the pH=1-3, concentrate under reduced pressure to remove most of the water, add 35 ml of ethanol, Cool down to 10°C, filter, and concentrate the filtrate to dryness to obtain 15 g of 1-bromo-3-propanesulfonic acid.
[0038] ③The 1-bromo-3-propanesulfonic acid obtained in the previous step was subjected to a lactonization reaction at 140-200°C under high vacuum (-0.1MPa), and the distillate was received at the same time to obtain 8.1 g of 1.3-propane sultone. The total yield of ...
Embodiment 3
[0040] ①At room temperature, add 18.6g of sodium sulfite (1.0eq), 50g of 1,3-dichloropropane (3.0eq), and 200ml of ethanol into the reactor. After the addition, raise the temperature to 80°C for 16 hours, then cool down to 10°C , and filtered to obtain crude sodium 1-chloro-3-propanesulfonate (unreacted 1,3-dichloropropane is in the filtrate and can be recovered).
[0041] ②Put the sodium 1-chloro-3-propanesulfonate obtained in the previous step into the reaction flask, add 60 ml of water, add hydrochloric acid below 20 degrees to adjust the pH=1-3, concentrate under reduced pressure to remove most of the water, add 35 ml of ethanol, Cool down to 10°C, filter, and concentrate the filtrate to dryness to obtain 19 g of 1-chloro-3-propanesulfonic acid.
[0042] ③ The 1-chloro-3-propanesulfonic acid obtained in the previous step was subjected to a lactonization reaction at 140-200° C. under high vacuum (-0.1 MPa), and the distillate was received at the same time to obtain 12.4 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com