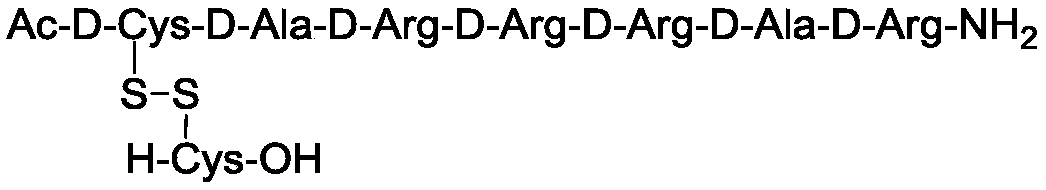Method for preparing AMG416 by combination of solid and liquid phase
A solid-liquid phase and solid-phase carrier technology, applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of reduced product purity and yield, cumbersome post-processing, easy cleavage of disulfide bonds, etc. To achieve the effect of reducing the increase of by-products, avoiding cumbersome post-processing, and reducing adverse factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
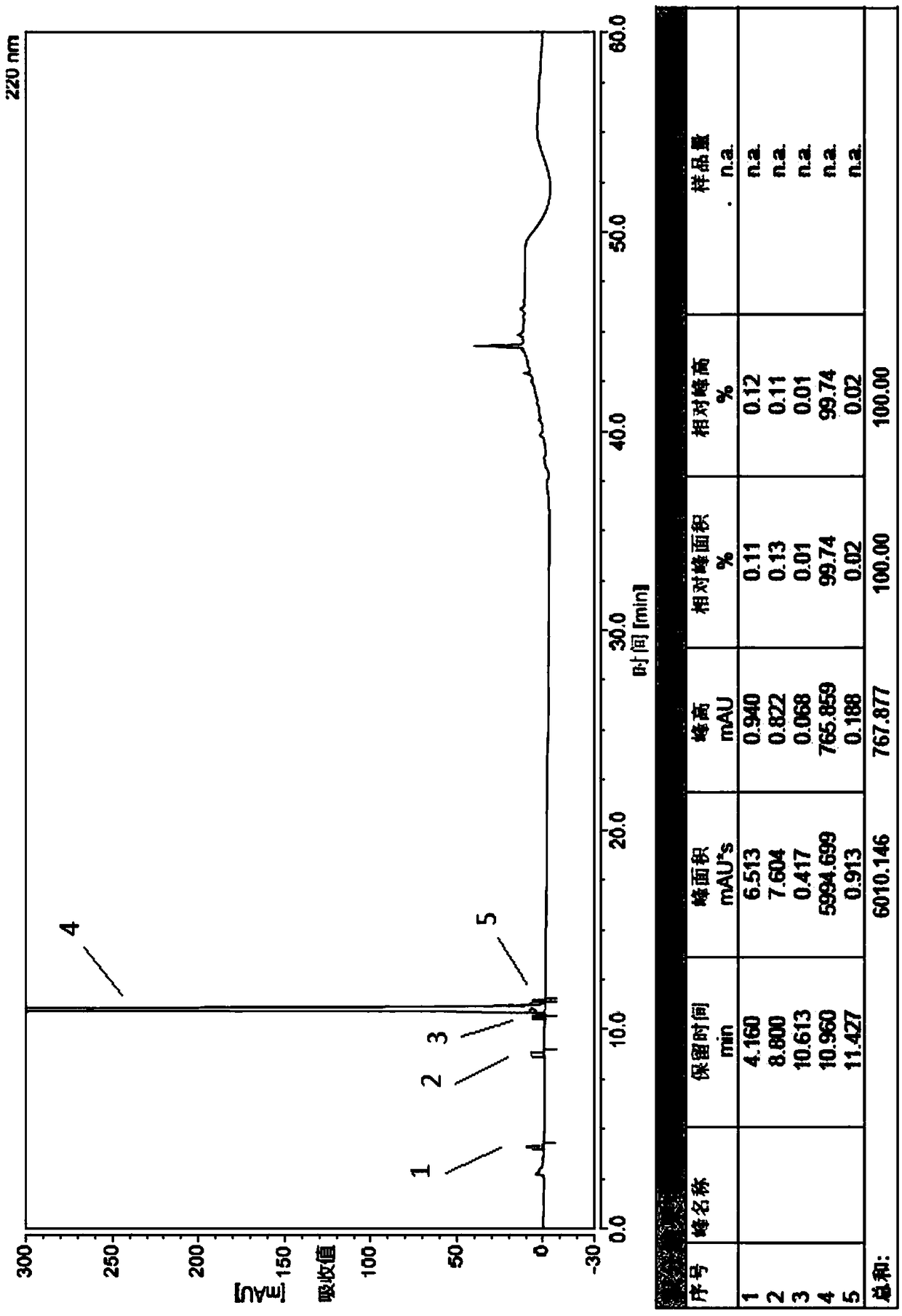
Examples
Embodiment 1
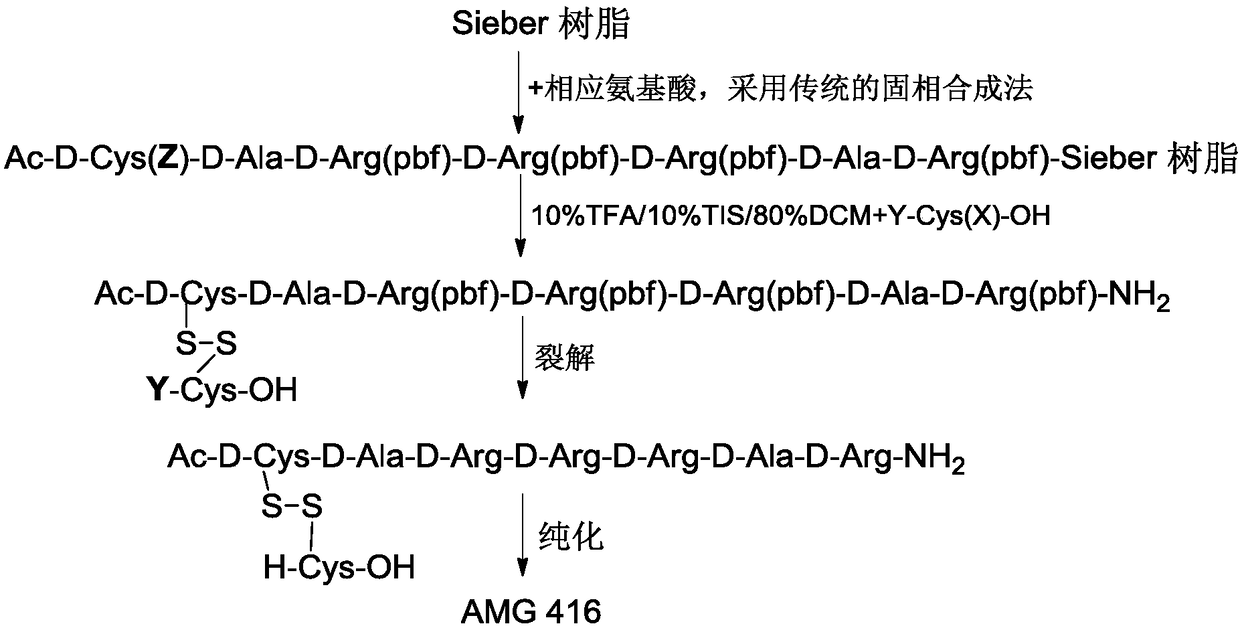
[0048] (1) Synthesis of peptide backbone
[0049] Weigh Sieber resin (5g, substitution value 0.69mmol / g resin) and join in the polypeptide reactor, add 50ml of DCM to wash and swell the resin; Use 20% piperidine / DMF solution to remove the Fmoc protecting group on the resin; DMF Wash the resin, remove Fmoc by-products and residual piperidine, ninhydrin detection (Kaiser Test), resin blue, complete deprotection;
[0050] Weigh Fmco-D-Arg(pbf)-OH (3eq, 6.7g), Cl-HoBt (3eq, 1.8g), HBTU (2.9eq, 3.8g) dissolved in 35ml DMF, then add DIEA (3.3eq, 1.9ml) was stirred, the reaction was detected by ninhydrin, filtered, washed with DMF, and detected by ninhydrin, the resin was colorless and transparent, and the condensation reaction was complete. Add 20% piperidine / DMF solution to remove the Fmoc protecting group on the resin, wash the resin with DMF, remove Fmoc by-products and residual piperidine, detect ninhydrin, the resin is blue, and the deprotection is complete.
[0051] Accordin...
Embodiment 2
[0067] (1) Synthesis of peptide backbone
[0068] Take Sieber resin (5.0g, substitution value 0.69mmol / g resin) and join in the polypeptide reactor, add 50ml DCM to wash and swell the resin, add 20% piperidine / DMF solution after filtering to remove the Fmoc protecting group; then add DMF washing resin, ninhydrin detection (Kaiser Test), resin blue, deprotection is complete.
[0069] Weigh Fmco-D-Arg(pbf)-OH (3eq, 6.7g), Oxyma (4.5eq, 2.2g), dissolve in 35ml DMF, then add DIC (6eq, 3.2ml), stir and add to the above polypeptide In the reactor, react at room temperature, take a small sample during the reaction, use ninhydrin to monitor the completion of the condensation, filter, wash the resin with DMF, and detect the ninhydrin, the resin is colorless and transparent, and the condensation reaction is complete. Add 20% piperidine / DMF solution to remove the Fmoc protecting group, wash the resin with DMF, detect ninhydrin, the resin is blue, and the deprotection is complete.
[00...
Embodiment 3
[0085] (1) Synthesis of peptide backbone
[0086] Take Sieber resin (2.0g, substitution value 0.69mmol / g resin) and join in the polypeptide reactor, add 20mlDCM to wash and swell the resin, add 20% piperidine / DMF solution after filtering to remove the Fmoc protecting group; then add DMF Wash the resin, remove Fmoc by-products and residual piperidine, ninhydrin detection (Kaiser Test), resin blue, complete deprotection;
[0087] Dissolve Fmco-D-Arg(pbf)-OH (3eq, 2.7g), Cl-HoBt (3eq, 0.7g) in 7ml DMF, then add DIC (3eq, 0.7ml), stir and add to the above polypeptide In the reactor, react at room temperature, take a small sample during the reaction, use ninhydrin to monitor the completion of the condensation, filter the resin, wash the resin with DMF, and detect the ninhydrin, the resin is colorless and transparent, and the condensation reaction is complete. Add 20% piperidine / DMF solution to remove the Fmoc protecting group, then use DMF to wash the resin to remove Fmoc by-produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com