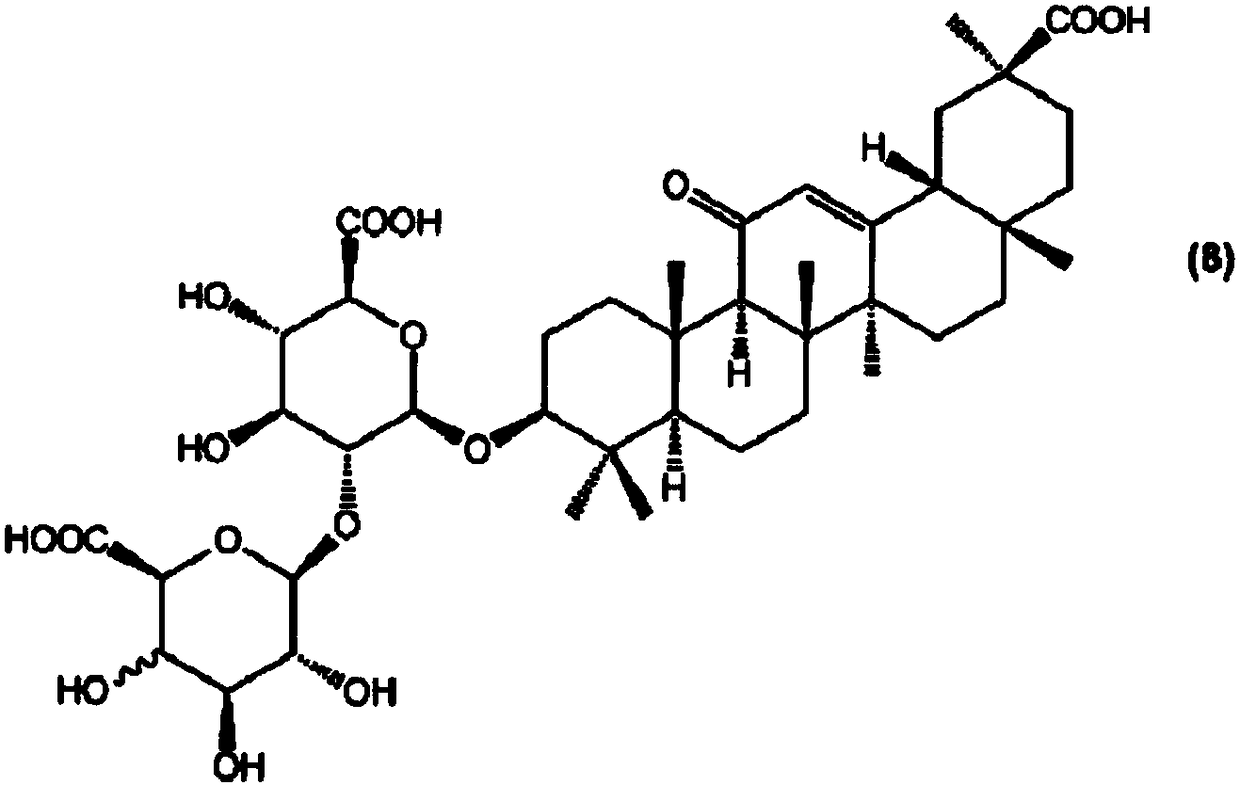
Manufacturing method of glycyrrhizic acid and D-galacturonic acid glycyrrhizic acid and intermediate used thereby
A manufacturing method and compound technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as complex procedures, low yield, and no reported synthesis method of galacturonic acid glycyrrhizic acid, etc., to achieve The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Manufacture of Glycyrrhizic Acid Galacturonate:
[0080] (1-1) Synthesis of Benzyl Glycyrrhetinate
[0081]
[0082] Glycyrrhetinic acid (compound (1')), 4.74g, 10.1mmol) and sodium bicarbonate (3.39g, 40.4mmol) were dissolved in dimethylformamide (25ml), and benzyl bromide (3.60ml, 30.3 mmol), stirred at 80°C for 2 hours. After cooling to room temperature, water and ethyl acetate were added. The aqueous layer was extracted with hexane: ethyl acetate = 4:1, the organic layer was washed with saturated brine, and dried over sodium sulfate. Sodium sulfate was removed by filtration, and the obtained filtrate was concentrated under reduced pressure. The obtained residue was purified by flash silica gel chromatography (hexane:ethyl acetate=5:1→3:1) to obtain the target compound (1-I) (MW560.81, 5.59g, 9.97 mmol) as a white powder ). The physicochemical properties of compound (1) are shown below.
[0083] 1 H-NMR (CDCl 3 ,600MHz)δ:0.69(1H,d,J=11.5Hz),0.73(3H,s),0.80...
Embodiment 2
[0137] Synthesis of glycyrrhizic acid;
[0138] (IVb) Glycosylation of Compound (3-I)
[0139]
[0140] Compound (3-I) (1.30 g, 1.31 mmol) produced in Example 1, and compound (4b-I) (phenyl 2,3,4,6-tetra-O-acetyl-1-sulfur N-iodosuccinimide (0.59 g, 2.62 mmol) was added to a solution of dichloromethane (50 ml) in substituted-β-D-glucopyranoside, 1.15 g, 2.61 mmol, and cooled to -60°C . A dichloromethane (1 ml) solution of trifluoromethanesulfonic acid (11.6 µl, 0.131 mmol) was added dropwise to the reaction solution over 10 minutes. Stirring was carried out while gradually raising the temperature to room temperature over 3.5 hours. Triethylamine was added to stop the reaction, and extracted with ethyl acetate. The organic layer was washed successively with saturated aqueous sodium bicarbonate solution, aqueous sodium thiosulfate solution, water, saturated aqueous sodium bicarbonate solution and saturated brine, and dried over sodium sulfate. After removing sodium sulfat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap