Substituted indole-2-carboxylic acid Bcl-2 small-molecule fluorescent probe and application thereof
A fluorescent probe, bcl-2 technology, applied in fluorescence/phosphorescence, luminescent materials, analytical materials, etc., can solve the problems of lack of design and synthesis methods, and few small molecule probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] The measurement of embodiment 1 optical activity
[0119] Table 1: Optical characteristics of probe molecules
[0120]
[0121] Note: All the above optical properties were measured in phosphate buffer at pH=7.4.
Embodiment 2
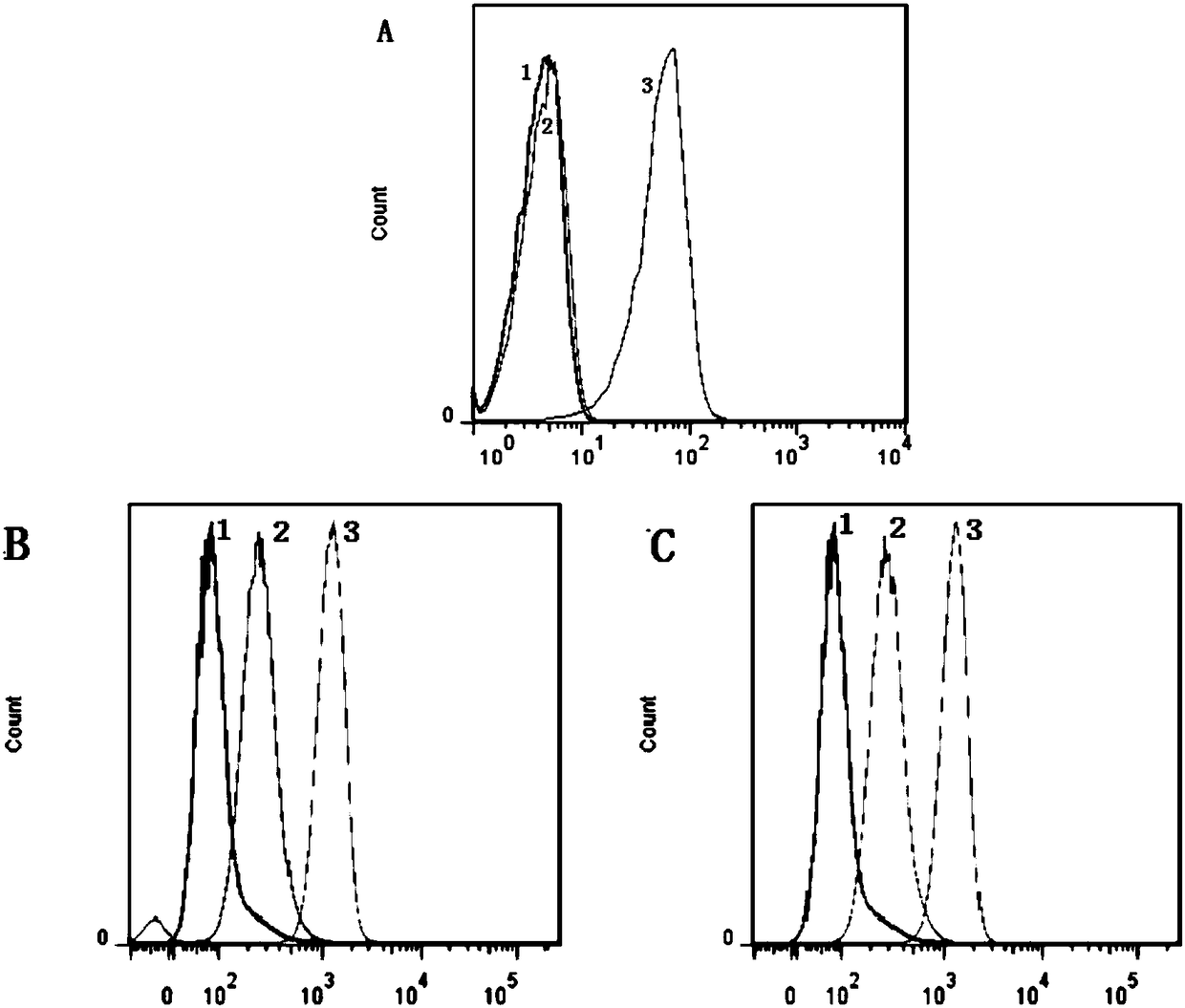
[0122] Embodiment 2: the mensuration of biological activity
[0123] Using gossypol (Gossypol) as a positive drug, FP or TR-FRET experiments were used to measure the binding activity of fluorescent probe molecules to Bcl-2 family proteins, and the results are shown in Table 2. The activities of the synthesized probe molecules L1, L3 and L3 were equivalent to those of the positive control gossypol, and the activity of the probe L4 was lower than that of the positive control gossypol.
[0124] Table 2: Affinity of probe molecules to Bcl-2 family proteins
[0125]
Embodiment 3
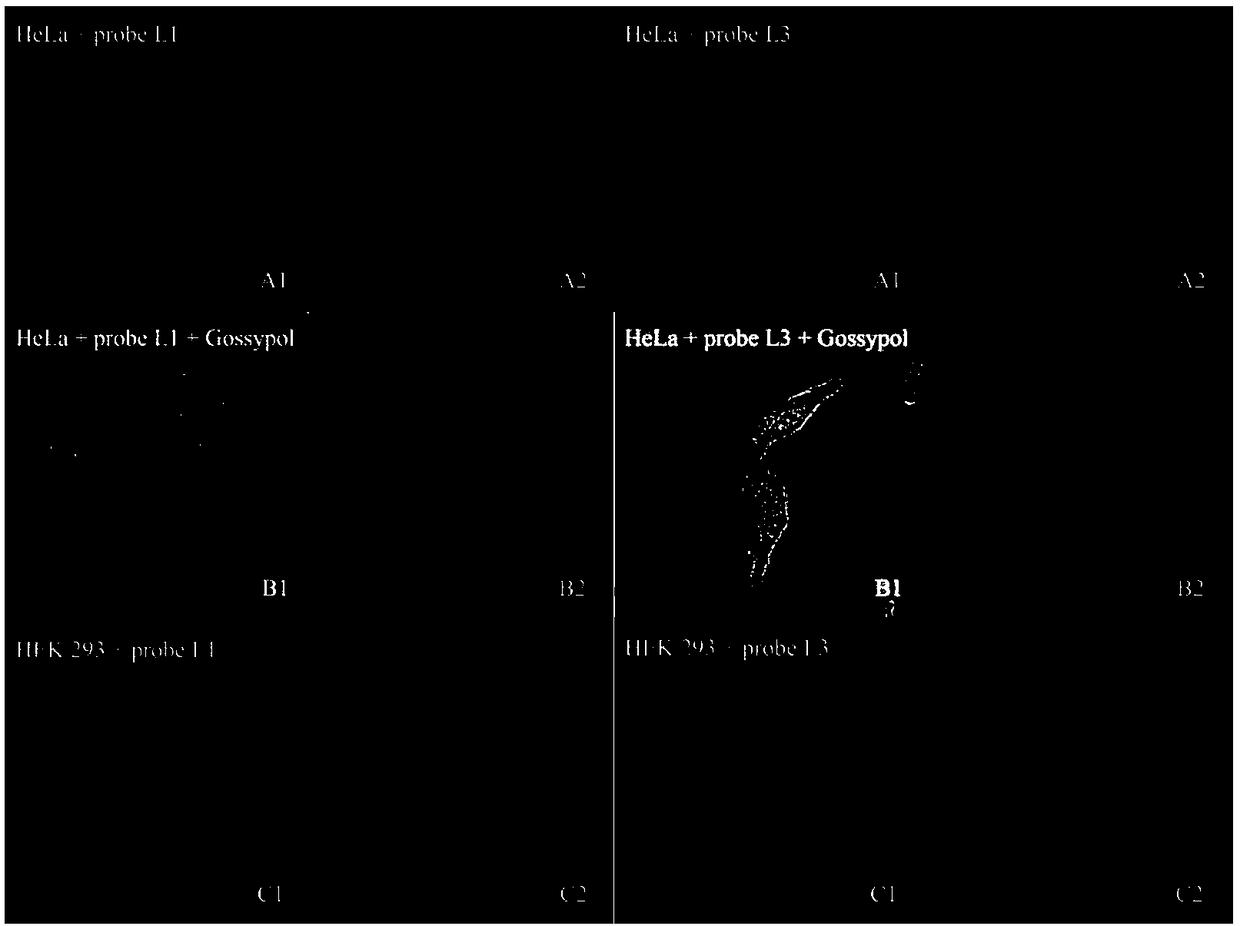
[0126] Example 3: Application of probe molecules in imaging HEK293 cells with high and low expression of Bcl-2
[0127] Probe molecules L1 and L3-L4 were used as research objects to investigate their application in cell imaging. Hela cells were selected as positive cells and HEK293 as negative cells. Under the same conditions, the inhibitor gossypol was added as a negative control. The specific steps are: Hela cells and HEK293 cells use DMEM medium containing 10% fetal bovine serum; 2 Culture in air and at 37°C. Before imaging, inoculate the cells in confocal small dishes, culture for 12-24 hours, suck off the medium, wash once with serum-free medium, and add probes L1-L4 respectively (Prepared in serum-free medium, the concentration is 1 μM); under the same conditions, another small dish was added with probes L1-L4 and inhibitor gossypol (prepared in serum-free medium, the concentration of L1 and L3 was 10 μM, gossypol Concentration of 10 μM; L4 concentration of 5 μM, gossyp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com