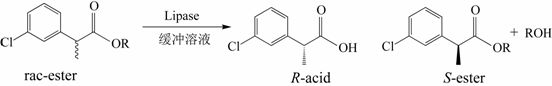
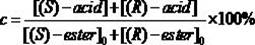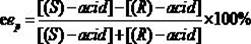A kind of stereoselective enzyme-catalyzed hydrolysis resolution method for enantiomers of 2-(3-chlorophenyl)propionic acid
A stereoselective, catalytic hydrolysis technology, applied in the direction of fermentation, etc., can solve the problems of low solubility and low conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 0.020 mmol of racemic heptyl 2-(3-chlorophenyl)propionate in a 25 mL reaction tube, and use 1 mL of disodium hydrogen phosphate / phosphate buffer solution (pH = 5.5) as the reaction medium, respectively Add 20 mg of different commercial lipases and react for 16 h at 600 rpm and 50 °C. After the reaction, the product was filtered and analyzed by high performance liquid chromatography. The results show that: when Pseudomonas cepacia lipase is used as a catalyst, it preferentially recognizes ( R )-2-(3-chlorophenyl) heptyl propionate, its ee p 98.16%, c was 11.09%.
Embodiment 2
[0028] Put 0.020 mmol of different kinds of racemic 2-(3-chlorophenyl) propionate in a 25 mL reaction tube, use 1 mL of disodium hydrogen phosphate / phosphate buffer solution (pH = 5.5) as the reaction medium, add 20 mg of Pseudomonas cepacia lipase was reacted for 15 h at 600 rpm and 40 °C. After the reaction, the product was filtered and analyzed by high performance liquid chromatography. The results show that: when the substrate is 2-(3-chlorophenyl) heptyl propionate, its ee p 98.20%, c was 7.26%.
Embodiment 3
[0030] Put 0.020 mmol of racemic heptyl 2-(3-chlorophenyl)propionate in a 25 mL reaction tube, use 1 mL of disodium hydrogen phosphate / phosphate buffer solution (pH = 5.5) as the reaction medium, add 20 mg Pseudomonas cepacia lipase was reacted for 4 h at 600 rpm and 70 °C. After the reaction, the product was filtered and analyzed by high performance liquid chromatography. The results show that: its ee p 98.82%, c was 10.31%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com