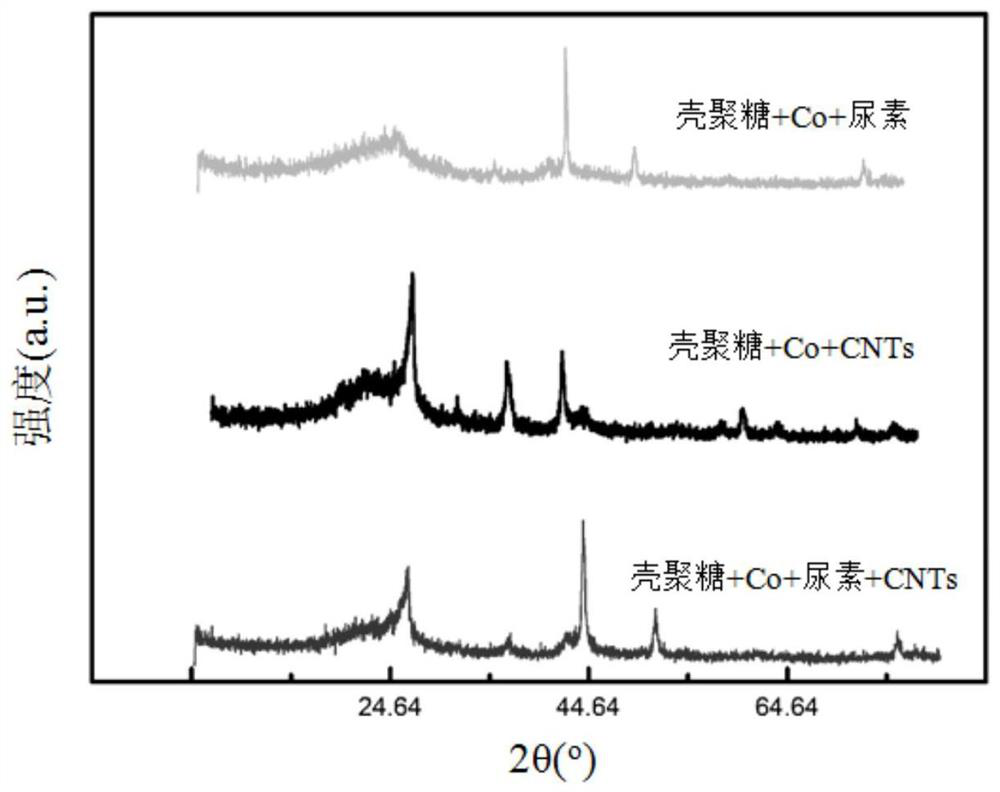
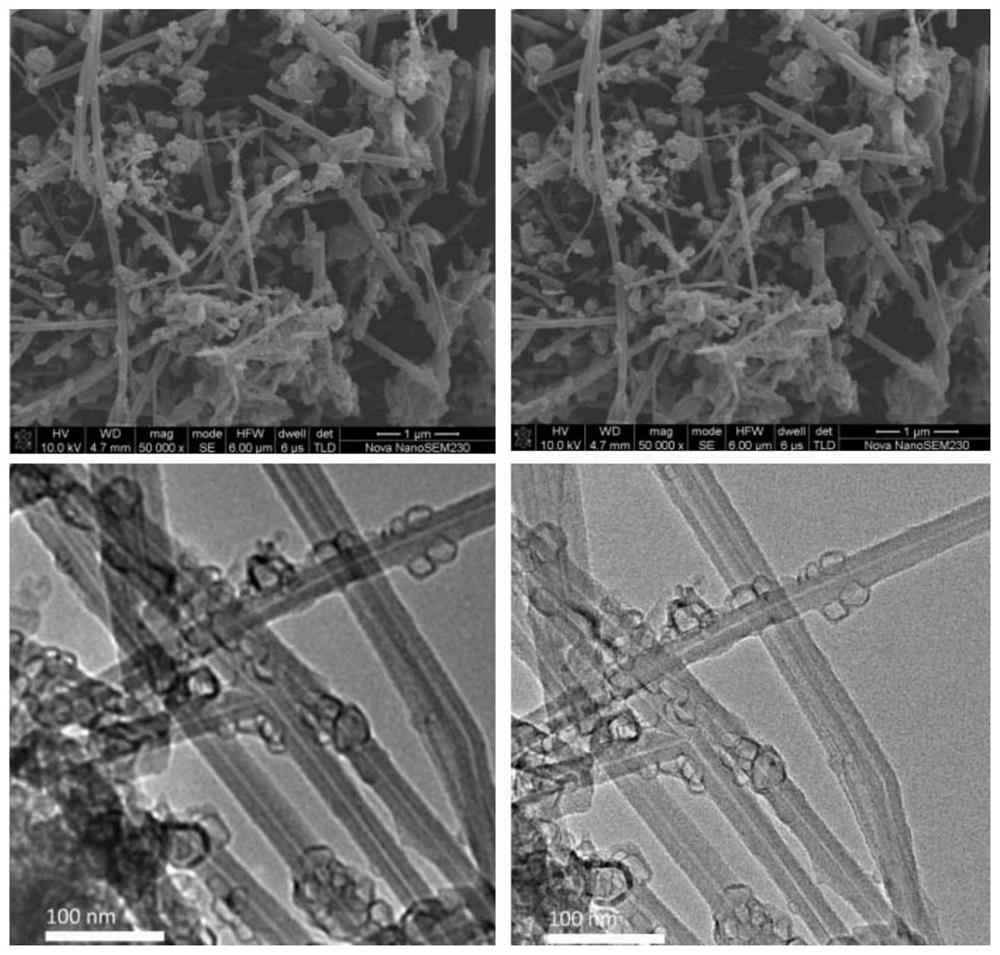
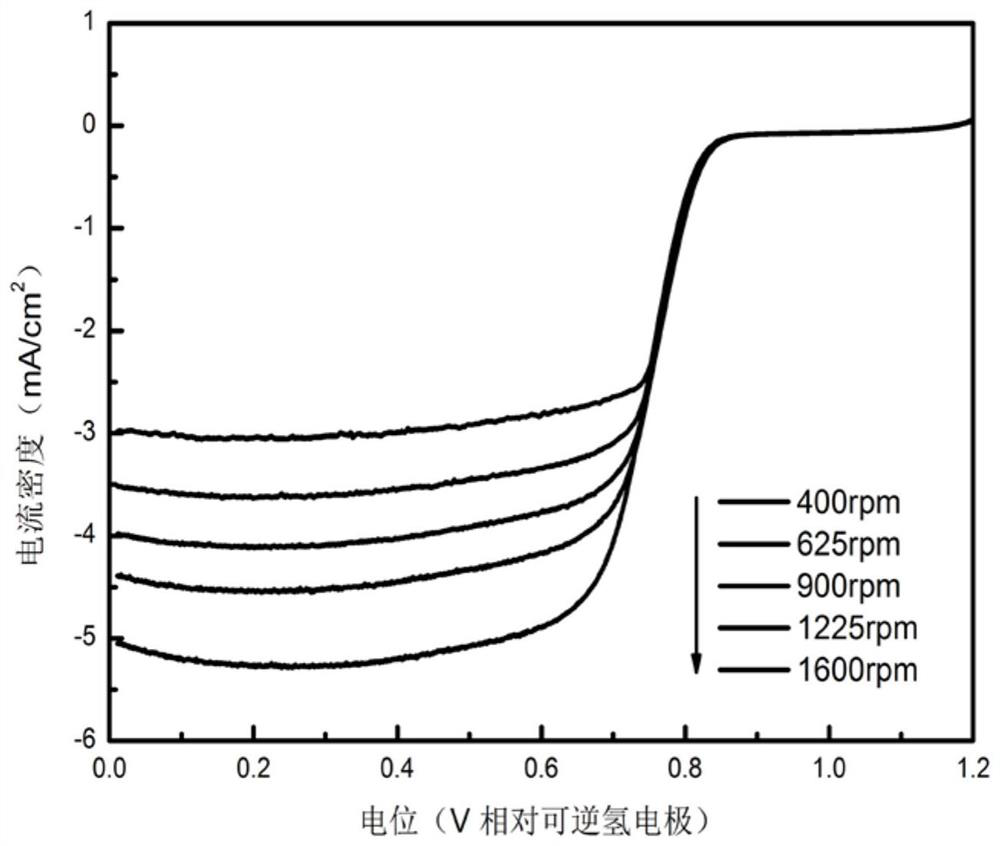
A kind of co-n-c composite catalyst and its preparation method and application
A composite catalyst, co-n-c technology, applied in the field of electrocatalysis, can solve the problems of difficulty in maintaining the stability of catalytic current, unstable graphitized C structure, and reduced catalyst activity, so as to achieve multiple electrochemical reaction active sites and enhance ORR Good dynamics and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Add 1 g of chitosan to 50 ml of distilled water and stir for 1 hour, add 10 drops of glacial acetic acid to dissolve the chitosan completely;
[0036] (2) Add 0.25g cobalt acetate and 1g urea, stir for 3h, make the cobalt ion fully carry out the chelation reaction with chitosan, and make the urea dissolve completely, move the mixed solution into a hydrothermal kettle with a magnetic stirring device, and the rotating speed is 300rpm , the hydrothermal temperature is 180°C, the reaction time is 8h, and the precursor solution is obtained;
[0037] (3) Add 0.2 g of carbon nanotubes to the precursor solution, stir for 1 h, put all the obtained solution into the liner of the reaction kettle, and put it in a drying oven for hydrothermal reaction. The hydrothermal temperature is 300 ° C, and the reaction time is 12 h. , the obtained product was placed in a freeze-drying box, dried for 72 hours, then put into a tube furnace (Ar gas) for calcining, the heating rate was 5°C / mi...
Embodiment 2
[0040] (1) Add 1 g of chitosan to 50 ml of distilled water and stir for 1 hour, add 10 drops of glacial acetic acid to dissolve the chitosan completely;
[0041] (2) Add 0.25g cobalt acetate and 1g urea, stir for 3h, make the cobalt ion fully carry out the chelation reaction with chitosan, and make the urea dissolve completely, move the mixed solution into a hydrothermal kettle with a magnetic stirring device, and the rotating speed is 300rpm , the hydrothermal temperature is 180°C, the reaction time is 8h, and the precursor solution is obtained;
[0042](3) Place the precursor solution in a freeze-drying oven, dry it for 72 hours, and then put it into a tube furnace (Ar gas) for calcining. The heating rate is 5°C / min. After rising to 900°C, it lasts for 2 hours. After cooling, the product is Sample 2 was obtained by grinding, and the obtained sample was used as a cathode material for an aluminum-air battery. The test method was as in Example 1. The electrochemical performance...
Embodiment 3
[0044] (1) Add 1 g of chitosan to 50 ml of distilled water and stir for 1 hour, add 10 drops of glacial acetic acid to dissolve the chitosan completely;
[0045] (2) Add 1 g of urea, stir for 3 hours to completely dissolve the urea, move the mixed solution into a hydrothermal kettle with a magnetic stirring device, the rotation speed is 300 rpm, the hydrothermal temperature is 180°C, and the reaction time is 8 hours to obtain a precursor solution;
[0046] (3) Add 0.2 g of carbon nanotubes to the precursor solution, stir for 1 h, put all the obtained solution into the liner of the reaction kettle, and put it in a drying oven for hydrothermal reaction. The hydrothermal temperature is 300 ° C, and the reaction time is 12 h. , the obtained product was placed in a freeze-drying box, dried for 72 hours, then put into a tube furnace (Ar gas) for calcining, the heating rate was 5°C / min, and continued for 2h after rising to 900°C, and the product was ground after cooling to obtain Sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com