Catalyst and preparation method and application in non-pressurized low-temperature synthesis ammonia thereof
A catalyst and catalytic activity technology, applied in chemical instruments and methods, physical/chemical process catalysts, ammonia compounds, etc., can solve the problems of preparation technology limitations, achieve the effect of changing reaction rate, improving performance and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
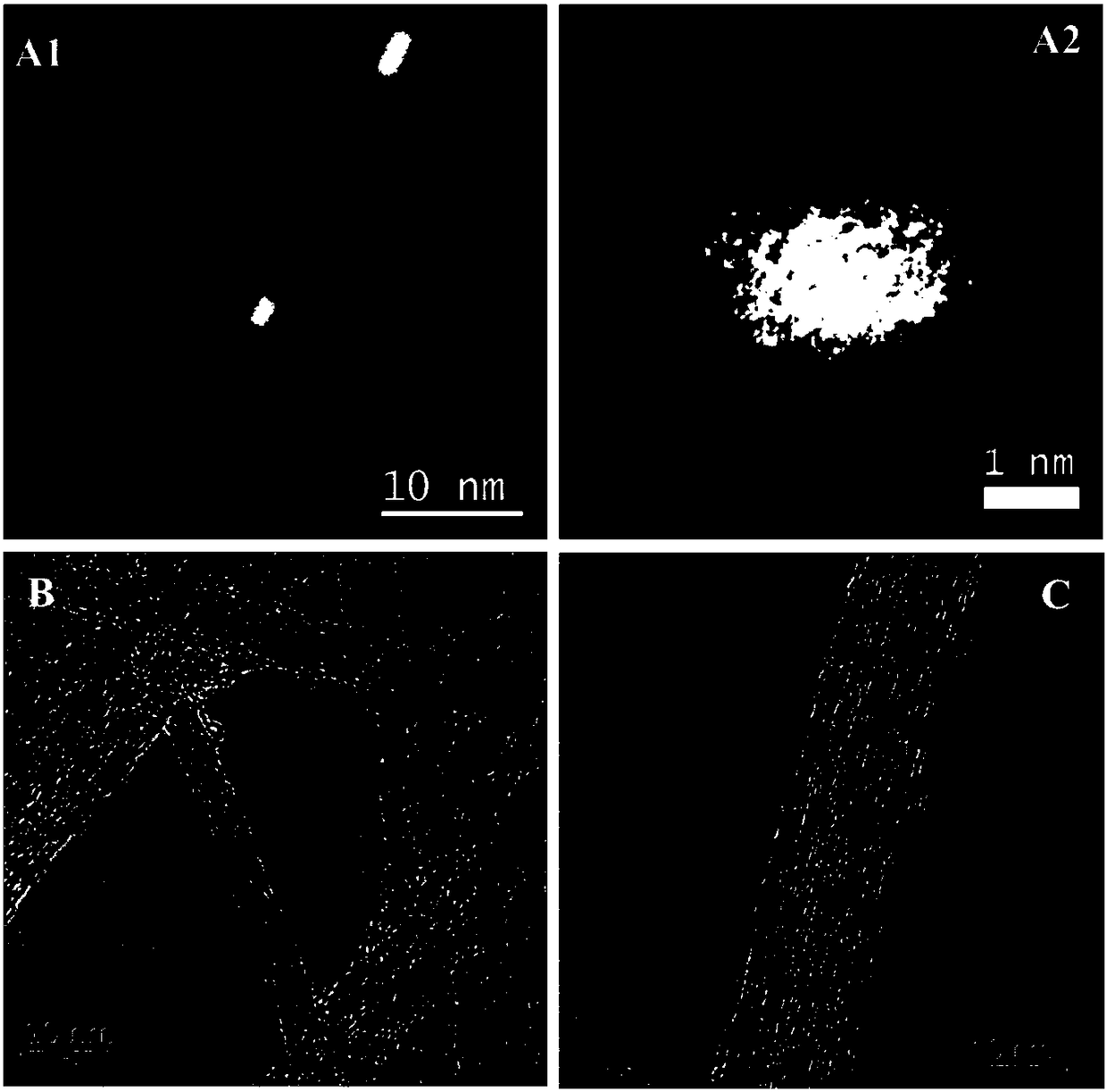
[0037] Put 4 parts of 60mg few-walled carbon nanotubes (TWCNTs) into four 100ml round bottom flasks respectively, add 60mL mixed acid (concentrated sulfuric acid:concentrated nitric acid=3:1) respectively, put them into an ultrasonic water bath at the same time, and sonicate for 5.5 hours, the temperature of the water bath was maintained at 40-50°C, and after ultrasonic treatment, 45 mL of each supernatant was taken, rinsed until neutral, and vacuum freeze-dried for 90 h. The dried sample was treated in argon at 1000°C for 4 hours, the flow rate of argon was 50mL / min, and it was taken out after cooling down to room temperature for use.
[0038] After truncated and purified, 220 mg of few-walled carbon nanotubes (s-TWCNTs) were obtained with a yield of up to 96%, and the truncated carbon tubes were evenly distributed in the length of 0.4-1.0 μm, the lumen was hollow, and the purity was higher than 95%.
Embodiment 2
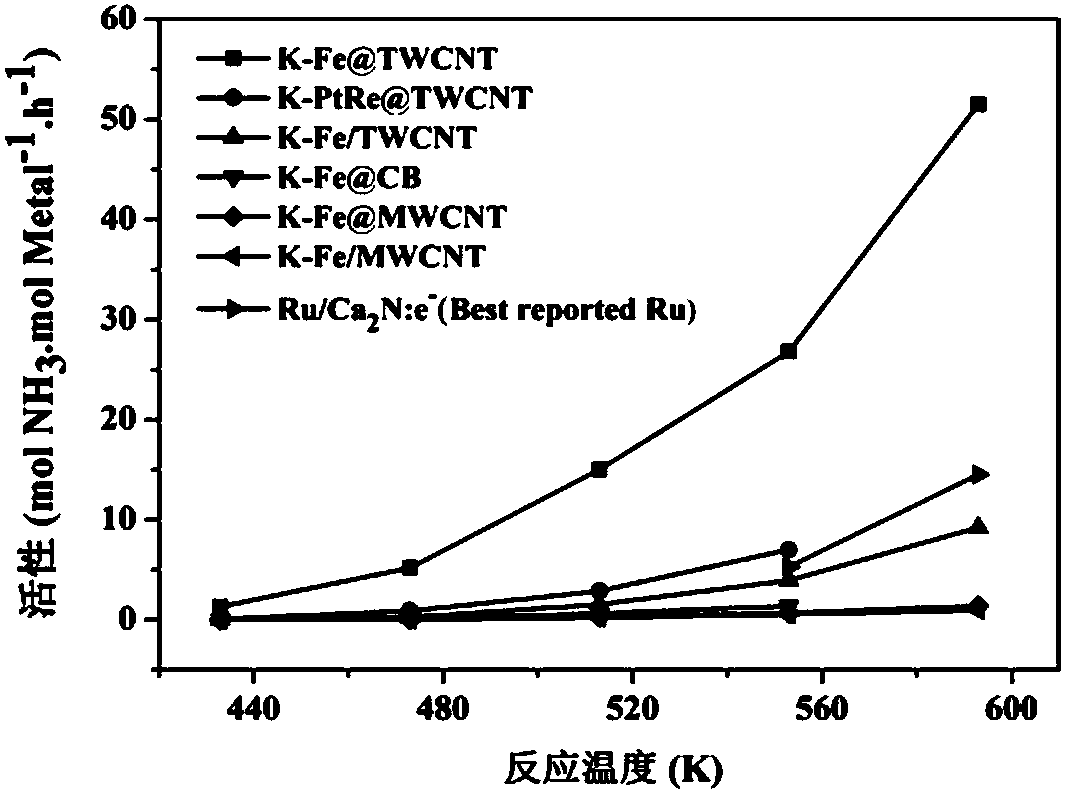
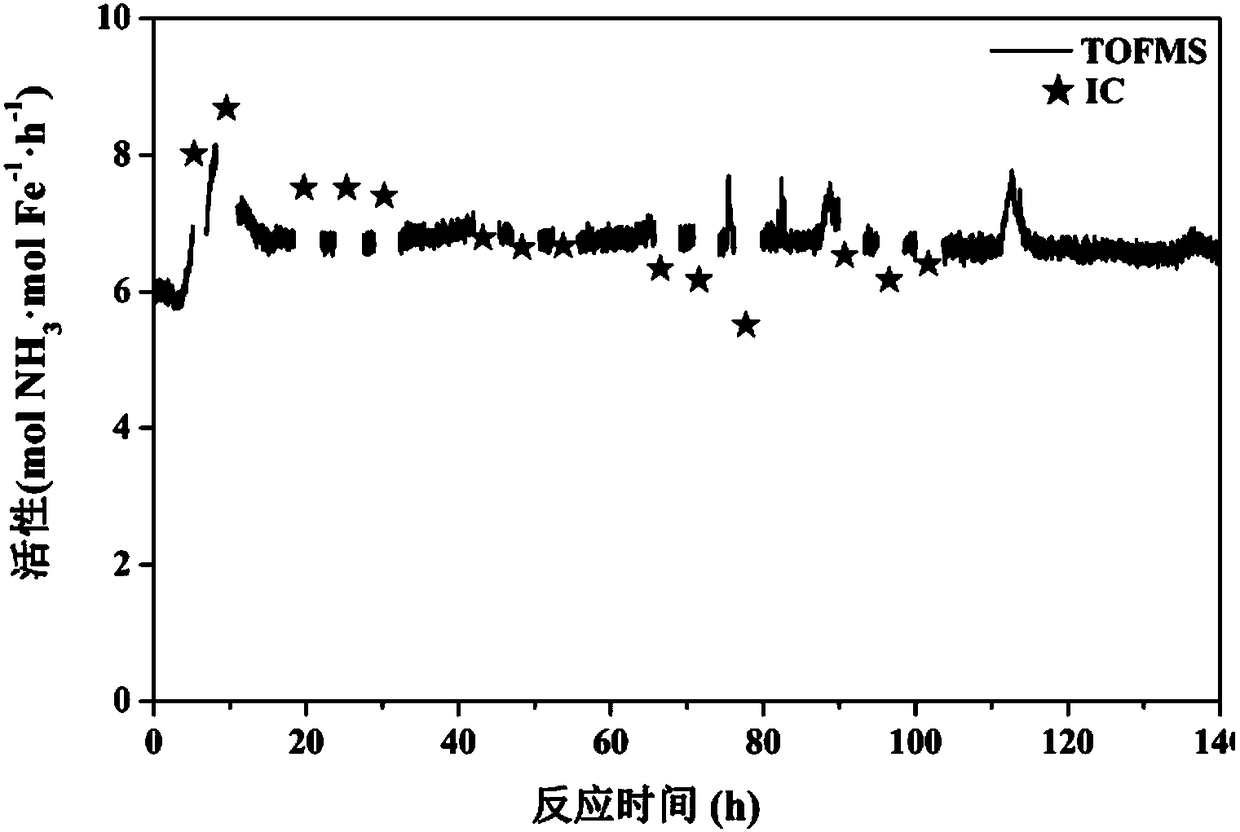
[0039] Example 2 Preparation of K-Fe@TWCNT catalyst
[0040] (1) Put 100 mg of truncated carbon nanotubes (s-TWCNTs) into a vacuum filling device, and put 38 mg of iron tricarbonyl cyclooctatetraenyl into the other end of the vacuum device. in 1×10 -4 Under the condition of Pa vacuum, the temperature of the device with carbon nanotubes was programmed to 450° C., the heating rate was 5° C. / min, and the temperature was kept at 450° C. for 16 hours. After cooling down, the carbon nanotubes and the precursor were mixed under vacuum, and then treated in an oven at 80°C for 72h.
[0041] (2) Fully rinse the filled sample with toluene, remove the residual Fe precursor outside the tube, filter, dry at 60°C overnight, oxidize with high pressure oxygen at 100°C for 24 hours, then stir with 2M nitric acid for 1 hour, filter , dried again at 60°C overnight, and reduced with hydrogen at 450°C for 5 hours to obtain a reduced 1wt.%Fe@TWCNTs catalyst.
[0042] (3) Seal the reduced catalyst...
Embodiment 3
[0044] Preparation of Example 3Cs-Ru@TWCNT Catalyst
[0045] (1) Put 100 mg of truncated carbon nanotubes (s-TWCNTs) into a vacuum-filled device, and put 2.5 mg of bis(2,4-dimethylpentadiene) ruthenium into the other end of the vacuum device. at 1.5 x 10 -4 Under the condition of Pa vacuum, the temperature of the device with carbon nanotubes was programmed to 450° C., the heating rate was 5° C. / min, and the temperature was kept at 450° C. for 16 hours. After cooling down, the carbon nanotubes and the precursor were mixed under vacuum, and then treated in an oven at 120°C for 48h.
[0046](2) Fully rinse the filled sample with toluene to remove the remaining Ru precursor outside the tube, filter, dry at 60°C overnight, and reduce with hydrogen at 450°C for 5 hours to obtain a reduced 1wt.%Ru@TWCNT catalyst.
[0047] (3) Seal the reduced catalyst and place it in an argon glove box, take 50 mg, add 26 μL of Cs metal, grind and mix evenly, and stir and activate at 100 °C for 12 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com