Preparation method of calcium magnesium ammonium nitrate
A technology of calcium and magnesium ammonium nitrate and ammonium nitrate is applied in the field of preparation of calcium and magnesium ammonium nitrate, can solve problems such as unfavorable large-scale production, high cost, environmental hazards, etc., and achieves solutions to land occupation and environmental pollution, good economic benefits, The effect of improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
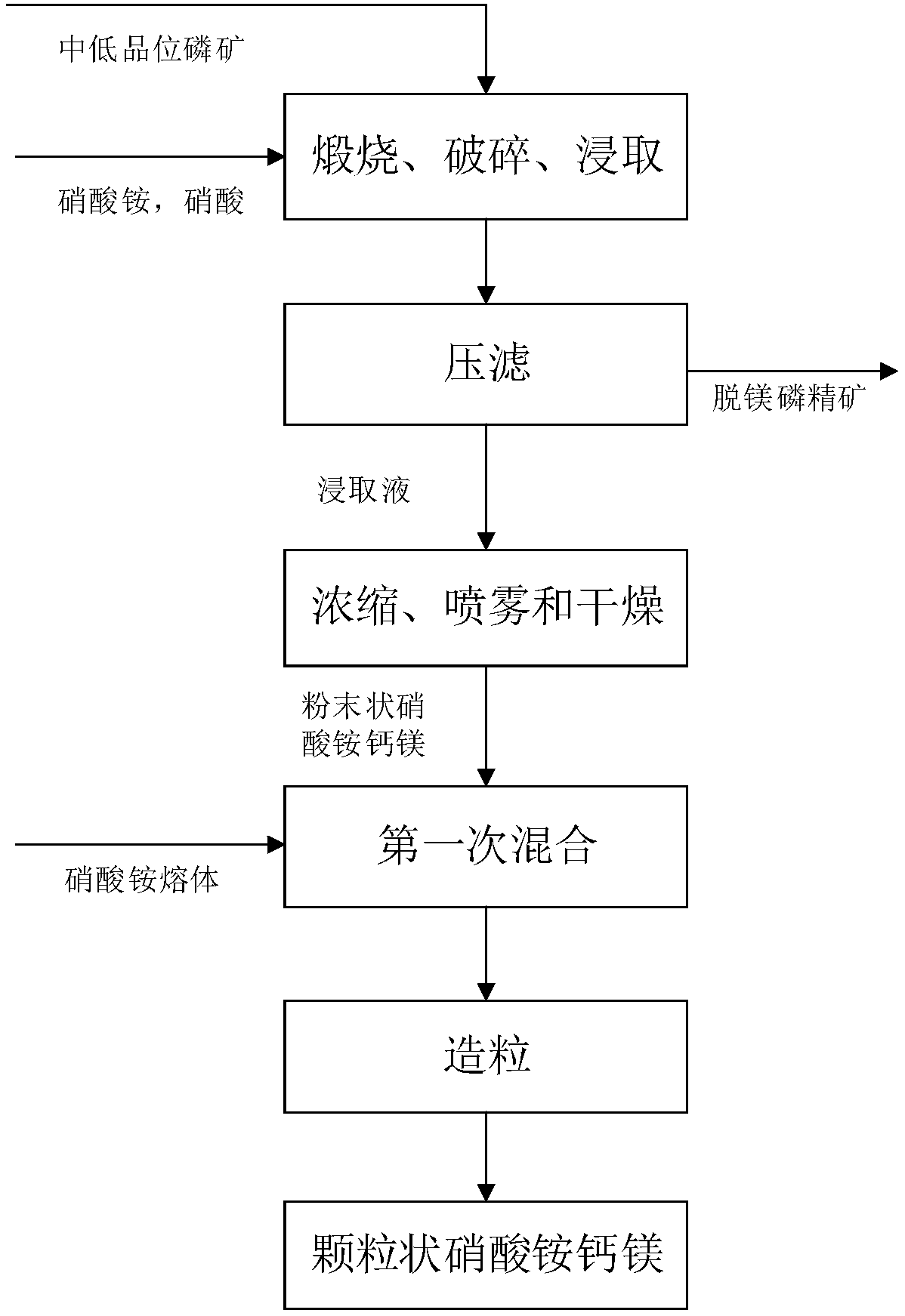
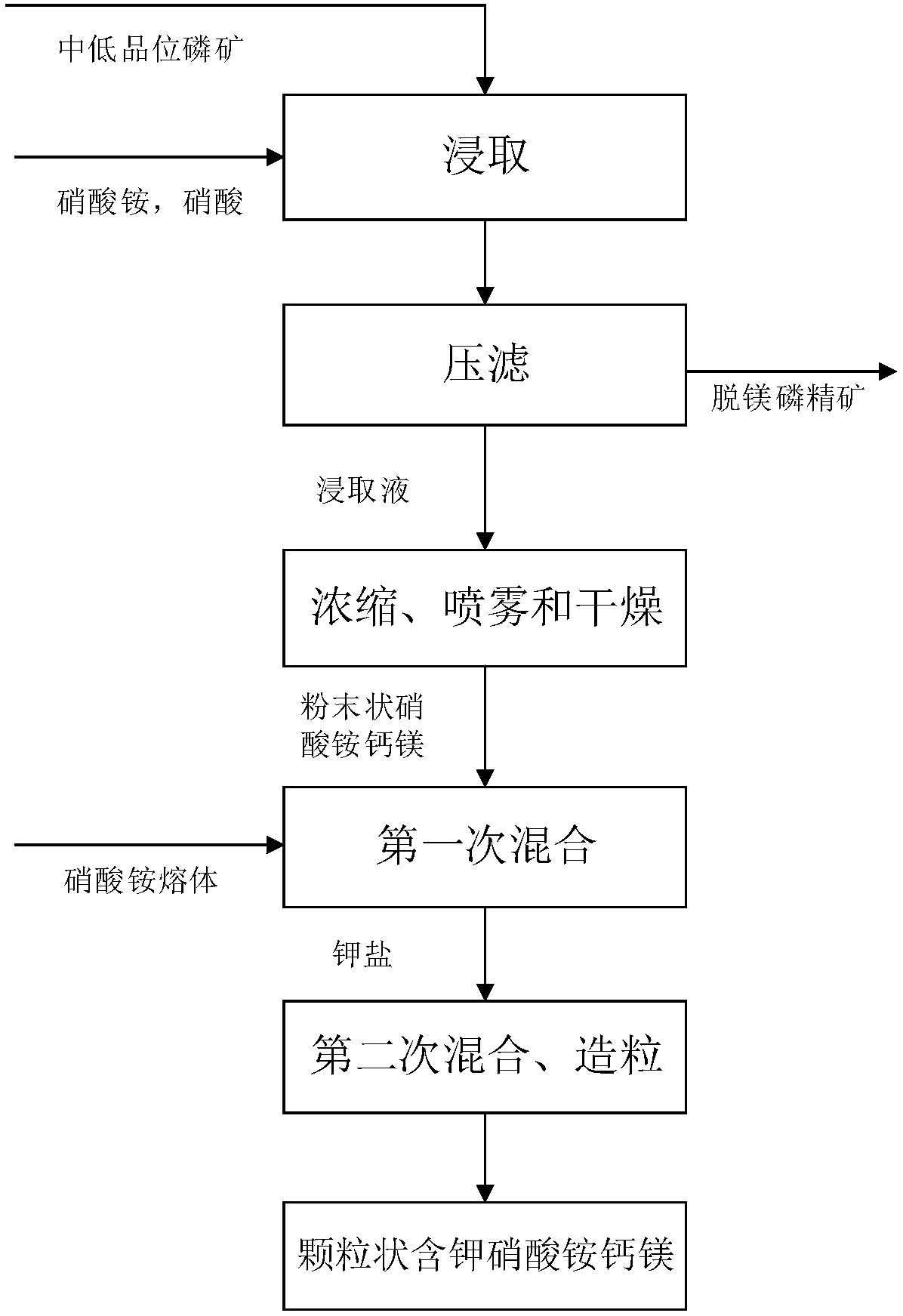
[0028] A kind of preparation method of ammonium nitrate calcium magnesium that the embodiment of the present invention provides, comprises the following steps:
[0029] Step 1: Calcining and crushing medium and low-grade phosphate rocks in sequence, and then adding the crushed medium and low-grade phosphate rocks to a solution containing ammonium nitrate and nitric acid for leaching and pressure filtration to obtain demagnesified phosphate concentrate and leachate;
[0030] First, the medium and low-grade phosphate rocks are sequentially calcined and crushed and then added to a solution containing ammonium nitrate and nitric acid. The ammonium nitrate and nitric acid solution reacts with the calcium oxide and magnesium oxide in the medium and low-grade phosphate rocks to form Magnesium nitrate, calcium ammonium nitrate and magnesium ammonium nitrate leaching solution, while the phosphorus in the middle and low grade phosphate rock still exists in the form of calcium fluorophos...
Embodiment 1
[0051] Take 40kg of medium and low-grade phosphate rock, and sequentially calcine and crush the medium and low-grade phosphate rock. During the calcination process, the calcination temperature is 900°C and the calcination time is 3h. The particle size of the crushed medium-low grade phosphate rock is 0.1-0.8mm. Then add the broken medium and low-grade phosphate rock into 20kg of solution containing ammonium nitrate and nitric acid for leaching. The mass ratio of ammonium nitrate and nitric acid is 4:1. During the leaching process, the leaching temperature is 65°C. The leaching time is 2.5 hours, and the pH value of the leaching solution is 4.0. After leaching, press filter, wash, and dry to obtain demagnesium phosphorus concentrate and leaching solution.
[0052] The leaching solution is concentrated and spray-dried in sequence to obtain powdered ammonium calcium magnesium nitrate. During the concentration process, the concentration temperature is 170° C., and the concentrat...
Embodiment 2
[0058] Take 40kg of medium and low-grade phosphate rock, and sequentially calcine and crush the medium and low-grade phosphate rock. During the calcination process, the calcination temperature is 920°C and the calcination time is 3h. The particle size of the crushed medium-low grade phosphate rock is 0.2-0.7mm. Then add the broken medium and low-grade phosphate rock to 12kg of solution containing ammonium nitrate and nitric acid for leaching. The mass ratio of ammonium nitrate and nitric acid is 3:1. During the leaching process, the leaching temperature is 75°C. The leaching time is 1 hour, and the pH value of the leaching solution is 5.5. After leaching, press filter, wash, and dry to obtain demagnesium phosphorus concentrate and leaching solution.
[0059] The leaching solution is concentrated and spray-dried in sequence to obtain powdered ammonium calcium magnesium nitrate. During the concentration process, the concentration temperature is 180°C, and the concentration tim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com