Uses of Tocotrienol Derivatives
A technology of use and medicine, applied in the field of use of tocotrienol derivatives, can solve the problems of increasing the risk of secondary bone marrow disease in the later stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: (2,8-dimethyl-2R-((3E,7E)-4,8,12-trimethyltridecyl-3,7,11-trienyl)benzene Preparation of the sodium salt of dihydropyran-6-yloxy) phosphate (i.e. the sodium salt of δ-tocotrienol phosphate, referred to as δ-T3HP) and confirm
[0059] Structural formula:
[0060]
[0061] Reaction equation:
[0062]
[0063] Step a:
[0064] Take 15g of δ-tocotrienol in a 250ml round bottom flask, add 45ml of toluene to dissolve, then add 6ml of pyridine, stir and cool to 0-5°C. Slowly add 4.5ml of phosphorus oxychloride dropwise. After the dropwise addition, move to room temperature and stir for 3h.
[0065] Step b:
[0066]Afterwards, the above reaction system was cooled to below 0°C, and 60ml of distilled water was slowly added. After the dropwise addition, heated to reflux for 4h.
[0067] stepc:
[0068] Add 100ml each of toluene and distilled water to the above reaction solution, shake well and let stand to separate layers. The organic layer was trans...
Embodiment 2
[0071] Example 2: 2,2-Dimethyl-(2R-(4R,8R,12-trimethyltridecyl)chroman-6-yloxy base) disodium phosphate (that is, the sodium salt of δ-tocopheryl phosphate, referred to as δ-THP) preparation and confirmation
[0072] Structural formula:
[0073]
[0074] Reaction equation:
[0075]
[0076] Step a:
[0077] Take 15g of δ-tocopherol in a 250ml round bottom flask, add 45ml of toluene to dissolve, then add 6ml of pyridine, stir and cool to 0-5°C. Slowly add 4.5ml of phosphorus oxychloride dropwise. After the dropwise addition, move to room temperature and stir for 3h.
[0078] Step b:
[0079] Afterwards, the above reaction system was cooled to below 0°C, and 60ml of distilled water was slowly added. After the dropwise addition, heated to reflux for 4h.
[0080] stepc:
[0081] Add 100ml each of toluene and distilled water to the above reaction solution, shake well and let stand to separate layers. The organic layer was transferred to a rotary evaporator, and th...
Embodiment 3
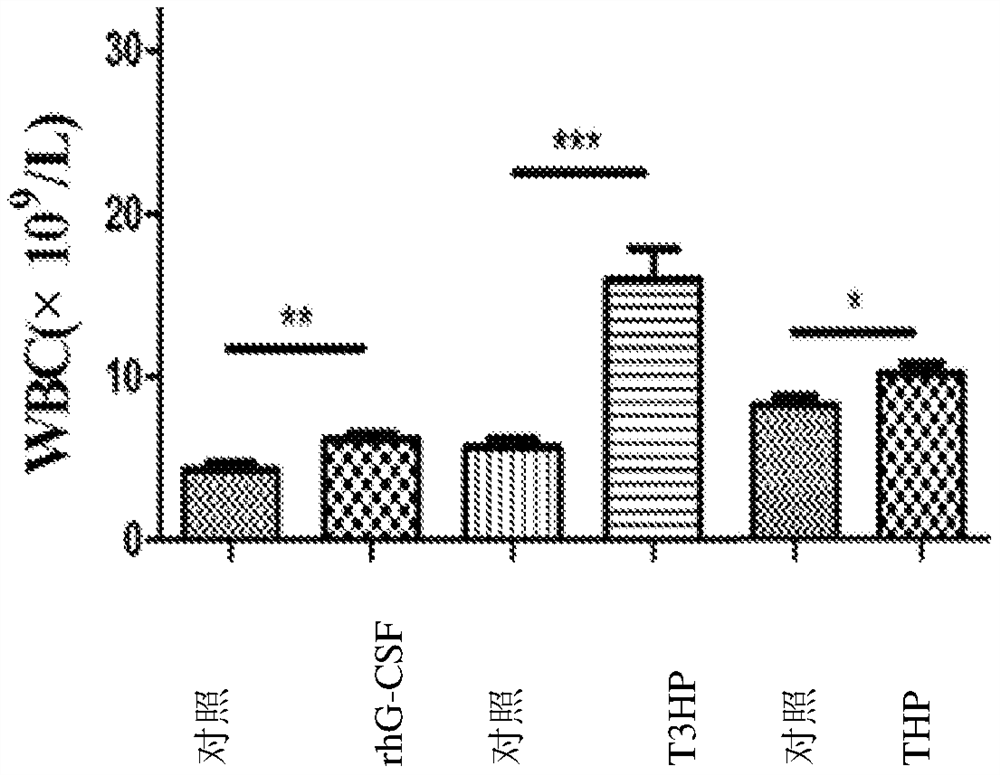
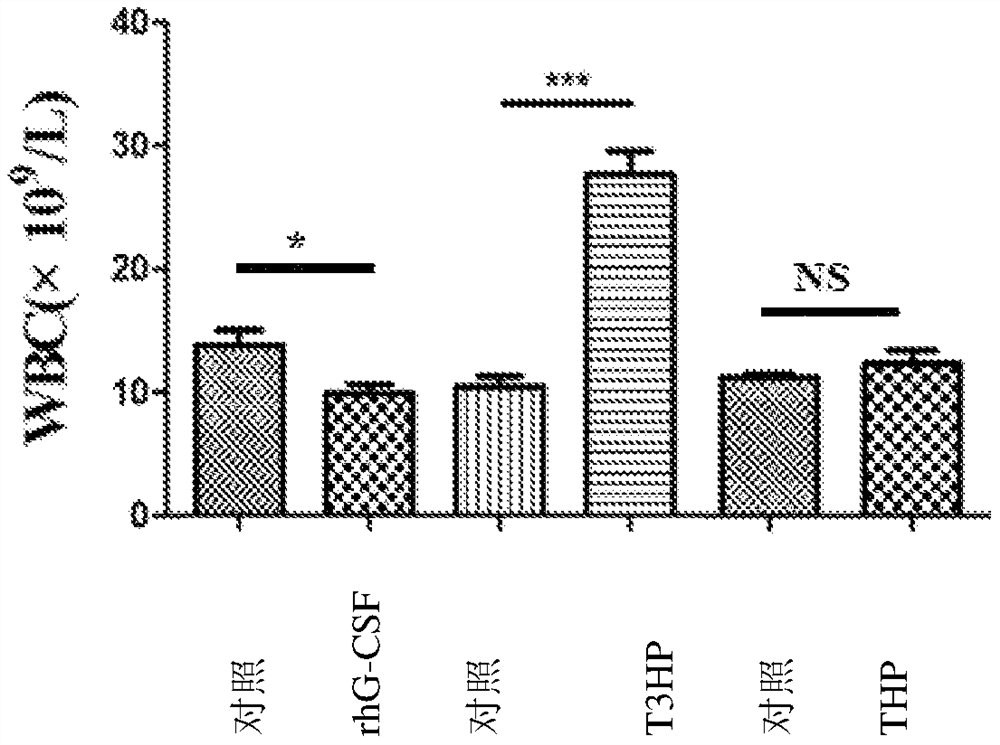
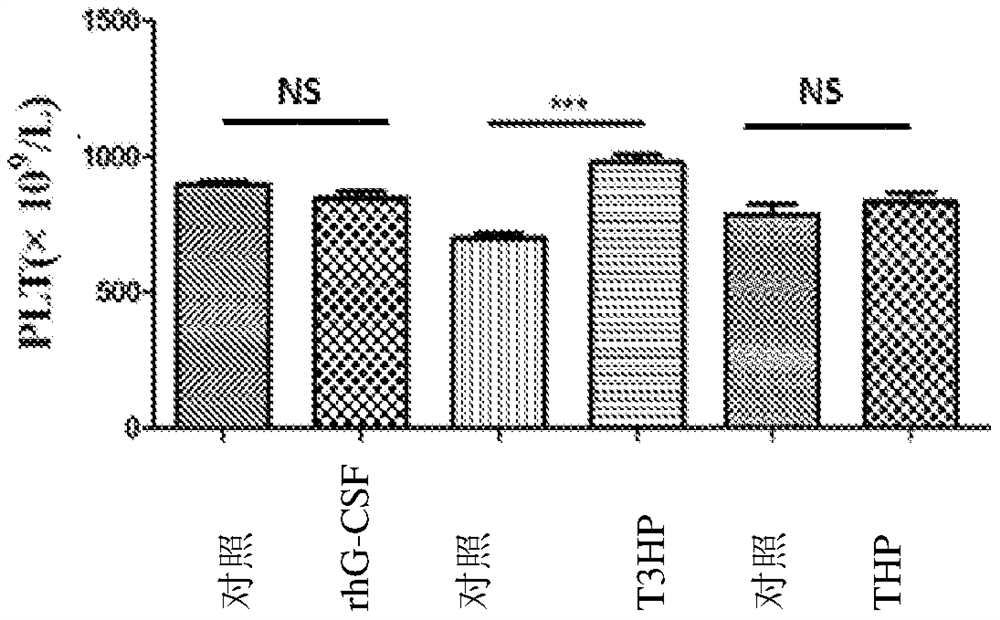
[0084] Example 3: Effects of δ-T3HP and δ-THP on the Number of Peripheral Blood Images in Cyclophosphamide Chemotherapy Mice
[0085] 3.1 Materials and methods
[0086] 3.1.1 Main reagents and instruments
[0087] Cyclophosphamide was purchased from Jiangsu Hengrui Pharmaceutical Co., Ltd. MEK-7222K automatic blood cell analyzer and peripheral blood detection diluent were purchased from Nihon Kohden Kogyo Co., Ltd.
[0088] 3.1.2 Experimental animals and grouping
[0089] The experiment used SPF grade C57BL / 6J male mice aged 6-8 weeks, weighing (22.6±0.89) g, purchased from Beijing Huafukang Biotechnology Co., Ltd.
[0090] 3.1.3 Chemotherapy conditions
[0091] Cyclophosphamide is used as a chemotherapy drug: 11.25 mg / ml solution is prepared with 0.9% normal saline before use, and 100 mg / Kg per mouse is administered intraperitoneally in 200 μl.
[0092] 3.1.4 Grouping and administration method
[0093] The experiment consisted of rhG-CSF control group and treatment gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com