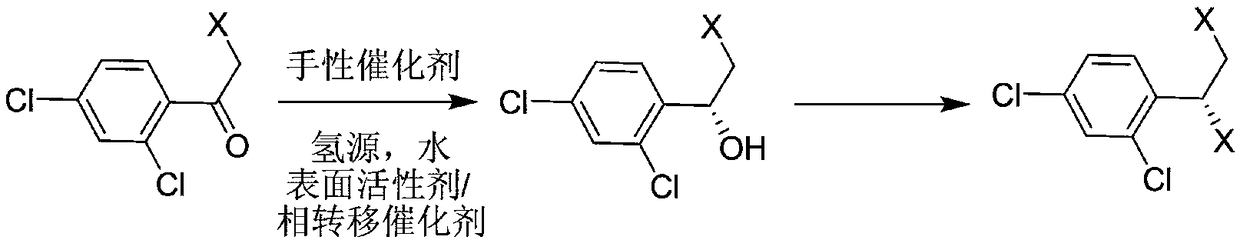
New synthesis method of luliconazole key chiral intermediate
A chiral intermediate, luliconazole technology, applied in the field of chemical synthesis, can solve the problems of large amount of carbonyl reductase T, unfavorable industrial production, high equipment cost, etc., and achieve easy industrial production, low product cost and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of (R)-2-chloro-1-(2,4-dichlorophenyl)ethanol
[0037] Under nitrogen protection, 0.31 grams of ruthenium complex precursor [Ru(p-cymene)Cl 2 ] 2 Add 0.67 g of optically pure (S,S)-N-p-methylbenzenesulfonyl-1,2-diphenylethylenediamine to a 250 ml reaction flask, add 100 ml of degassed water, and heat to 35 React at -40°C for 1 hour; then add 0.25 g of cetyltrimethylammonium bromide, 52 g of NaHCO 3 2H 2 O and 22 grams of ω-chloro-2,4-dichloroacetophenone, the reaction temperature is 25-35 ° C, and the temperature is maintained for 14 hours. After the reaction is completed, the product is extracted with n-hexane, and the extract is used without Dry over sodium sulfate, filter out sodium sulfate, and concentrate to dryness; add 55 ml of n-hexane and heat to dissolve, then cool down to room temperature for crystallization, filter, and dry to obtain (R)-2-chloro-1-(2,4 - 21.2 grams of dichlorophenyl) ethanol, optical purity 99.2%.
[0038] Catalyst recycling ...
Embodiment 2
[0045] Synthesis of (R)-2-chloro-1-(2,4-dichlorophenyl)ethanol
[0046] Under nitrogen protection, 0.15 grams of ruthenium complex precursor [Ru(p-cymene)Cl 2 ] 2 Add 0.34 g of optically pure (S,S)-N-p-methylbenzenesulfonyl-1,2-diphenylethylenediamine to a 150 ml reaction flask, add 60 ml of degassed water, and heat to 35 -40°C; after 1 hour of reaction, add 0.12 g of cetyltrimethylammonium bromide, 26 g of NaHCO 3 2H 2 O and 11 grams of ω-chloro-2,4-dichloroacetophenone, the reaction temperature is 25-35 ° C, keep the temperature for 12 hours; add n-hexane to extract the product, and the extract is dried with anhydrous sodium sulfate , filtered off sodium sulfate, concentrated to dryness, then added 30 ml of n-hexane and heated to dissolve, then lowered to room temperature for crystallization, filtered, and dried to obtain (R)-2-chloro-1-(2,4-dichlorobenzene Base) 10.6 grams of ethanol, optical purity 99.3%.
Embodiment 3
[0048] Synthesis of (R)-2-chloro-1-(2,4-dichlorophenyl)ethanol
[0049] Under nitrogen protection, 0.15 grams of ruthenium complex precursor [Ru(p-cymene)Cl 2 ] 2 Add 0.34 g of optically pure (S,S)-N-p-methylbenzenesulfonyl-1,2-diphenylethylenediamine to a 150 ml reaction flask, add 60 ml of degassed water, and heat to 35 -40°C; After 1 hour of reaction, add 0.54 g of sodium lauryl sulfate and 26 g of NaHCO 3 2H 2 O and 11 grams of ω-chloro-2,4-dichloroacetophenone, the reaction temperature is 25-35 ° C, keep the temperature for 12 hours, the reaction is complete; add n-hexane to extract the product, the extract is anhydrous Dry over sodium sulfate, filter out sodium sulfate, concentrate to dryness, add 30 ml of n-hexane and heat to dissolve, then cool down to room temperature for crystallization, filter, and dry to obtain (R)-2-chloro-1-(2,4- Dichlorophenyl) ethanol 10.5 grams, optical purity 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com