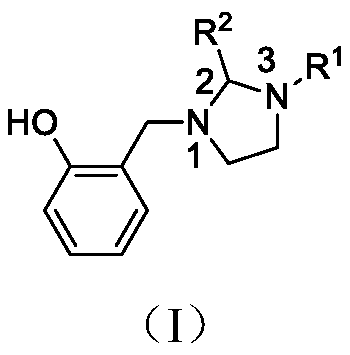
Aryl imidazoline compounds and their preparation methods and uses
A technology of aryl imidazoline and imidazoline, which is applied in the field of pesticides, can solve the problems of less research on the antibacterial activity of pesticides and no reports on the antibacterial activity of imidazoline compounds, and achieve good antibacterial activity, simple synthesis method, and cheap synthetic materials Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
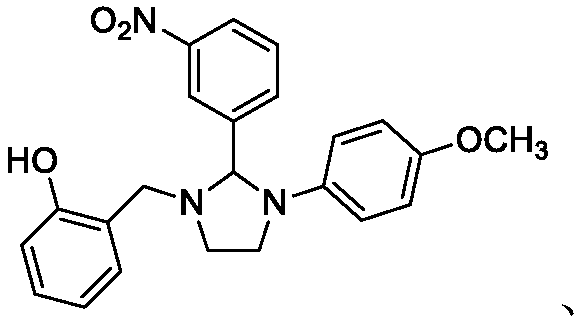
[0128] Example 1: Synthesis of 1-(2-hydroxybenzyl)-2-(3-nitrophenyl)-3-(4-methoxyphenyl)imidazoline.
[0129] Weigh N-(4-methoxyphenyl)-N'-(2-hydroxyphenyl)ethylenediamine 0.280g (1mmol), 3-nitrobenzaldehyde 0.212g (1.4mmol), La(SO 3 CF 3 ) 3 0.059g (0.10mmol) and 0.25g powder molecular sieve into a 100mL three-necked flask, and then add 30mL CHCl 3 Mixed solvent with cyclohexane (V:V=1:4), feed N 2 protective gas. Heated to 80°C and refluxed for 3h, and desolvated under reduced pressure after the reaction was complete. Extract with ethyl acetate (50mL×3 times), combine the organic layers, wash with distilled water (40mL×3) and saturated NaCl solution (40mL×3) successively, and then wash with saturated NaHSO 3 solution (40mL×3) was washed to remove excess aldehyde, and then washed with anhydrous Na 2 SO 4 The organic phase was dried, filtered with suction, precipitated under reduced pressure and subjected to column chromatography to obtain a red solid, melting point:...
Embodiment 2
[0131] Example 2: Synthesis of 1-(2-hydroxybenzyl)-2-(2-nitrophenyl)-3-(4-methoxyphenyl)imidazoline.
[0132] Weigh N-(4-methoxyphenyl)-N'-(2-hydroxyphenyl)ethylenediamine 0.276g (1mmol), 2-nitrobenzaldehyde 0.213g (1.4mmol), La(SO 3 CF 3 ) 3 0.059g (0.10mmol) and 0.25g powder molecular sieve into a 100mL three-necked flask, and then add 30mL CHCl 3 , into the helium protective gas. Heated to 85°C and refluxed for 1h, and desolvated under reduced pressure after the reaction was complete. Extract with ethyl acetate (50mL×3 times), combine the organic layers, wash with distilled water (40mL×3) and saturated NaCl solution (40mL×3) successively, and then wash with saturated NaHSO 3 solution (40mL×3) was washed to remove excess aldehyde, and then washed with anhydrous Na 2 SO 4 The organic phase was dried, filtered with suction, precipitated under reduced pressure and subjected to column chromatography to obtain a red solid with a melting point (mp): 143.1-143.3° C. and a ...
Embodiment example 3
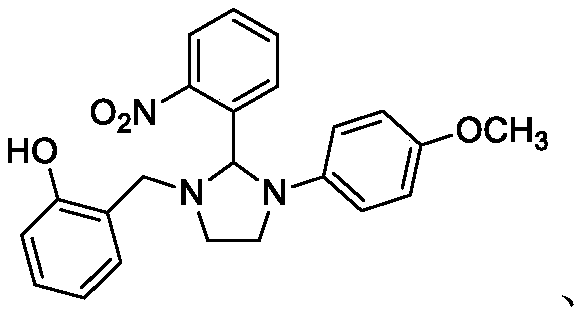
[0134] Example 3: Synthesis of 1-(2-hydroxybenzyl)-2-(4-nitrophenyl)-3-(3-tolyl)imidazoline.
[0135] Weigh N-(3-methylphenyl)-N'-(2-hydroxyphenyl)ethylenediamine 0.256g (1mmol), 4-nitrobenzaldehyde 0.214g (1.4mmol), La(SO 3 CF 3 ) 3 0.059g (0.10mmol) and 0.25g powder molecular sieve into a 100mL three-necked flask, then add 30mL tetrahydrofuran, and pass through N 2 protective gas. Heated to 90°C and refluxed for 8h, and desolvated under reduced pressure after the reaction was complete. Extract with ethyl acetate (50mL×3 times), combine the organic layers, wash with distilled water (40mL×3) and saturated NaCl solution (40mL×3) successively, and then wash with saturated NaHSO 3 solution (40mL×3) was washed to remove excess aldehyde, and then washed with anhydrous Na 2 SO 4 The organic phase was dried, filtered with suction, and precipitated under reduced pressure to obtain an orange solid by column chromatography, melting point (mp): 162.5-163.5°C, yield: 79.7%.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com