f-bpa and its intermediate synthesis method, intermediate and application
A F-BPA, chemical synthesis technology, applied in the field of F-BPA nucleophilic fluorination synthesis, can solve the problems of high manufacturing cost, low specific activity, and products with carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] According to one embodiment of the present invention, a kind of F-BPA nucleophilic fluorination synthetic method is provided, and the method comprises the following steps:
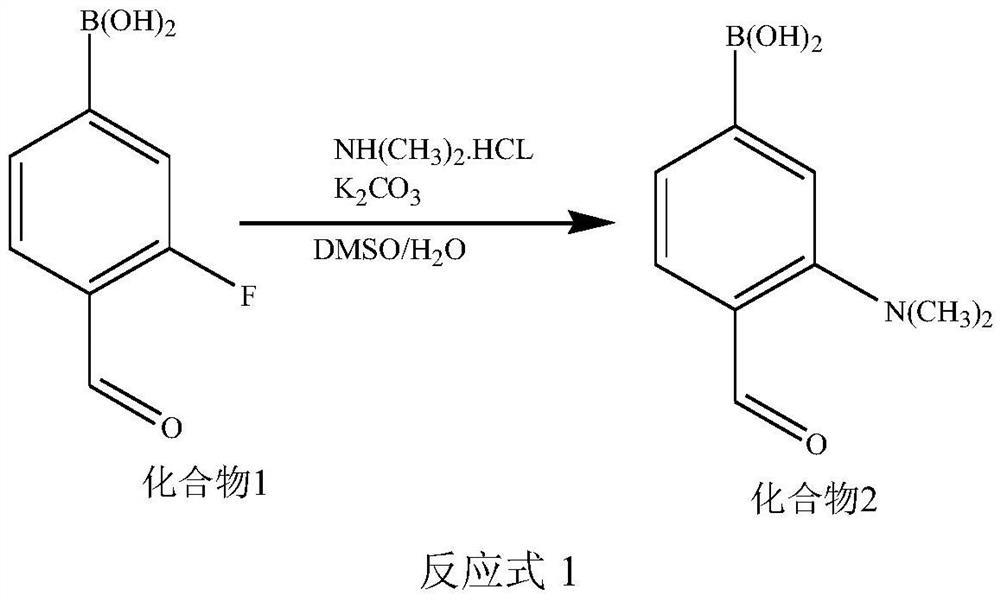
[0067] (1) Synthesis of Compound 2
[0068] Add compound 1 and dimethylammonium hydrochloride to a solvent mixed with dimethyl sulfoxide and water, and add K in more than 2 times 2 CO 3 , heated to reflux for 6h to 36h; wherein, compound 1: dimethylammonium hydrochloride: K 2 CO 3 The molar ratio is 1:1-5:1-5; the reaction and the structural formula of the compound 1 are shown in the reaction formula 1; after the reaction is completed, the compound 2 is obtained by separation and purification.
[0069]
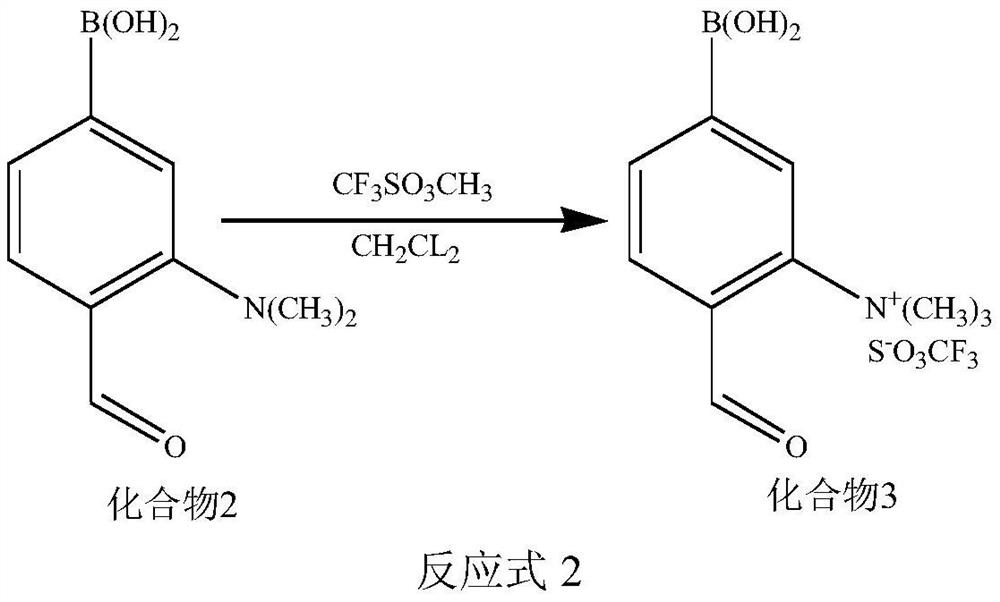
[0070] (2) Synthesis of compound 3
[0071] Under the protection of an inert gas, mix the dichloromethane solution of compound 2 with the dichloromethane solution of methyl-trifluoromethanesulfonate, and stir for 3h to 12h, wherein, compound 2: methyl-trifluoromethane The molar ratio of sulf...
Embodiment 1
[0121] This embodiment relates to the nucleophilic fluorination synthesis of F-BPA, and the synthesis process includes the following steps:
[0122] (1) Synthesis of compound 2
[0123] Add 0.84g compound 1, 0.82g dimethyl ammonium hydrochloride, 0.83g K 2 CO 3 , add a reaction solvent composed of 20mL dimethyl sulfoxide and 8mL water, reflux for 2h, add 0.55g K to the side port 2 CO 3 , continue the reflux reaction for 4h, lower the reaction solution to room temperature, stop the reaction, add the reaction solution to 40mL saturated K 2 CO 3 In the aqueous solution, the reaction solution is divided into two layers, separated by a separatory funnel with K 2 CO 3 The remaining reaction solution was extracted twice with 30 mL of diethyl ether, the diethyl ether layers were combined, washed once with 30 mL of water, and dried overnight with anhydrous magnesium sulfate. Anhydrous magnesium sulfate was removed by filtration, diethyl ether was rotary evaporated to dryness to ...
Embodiment 2
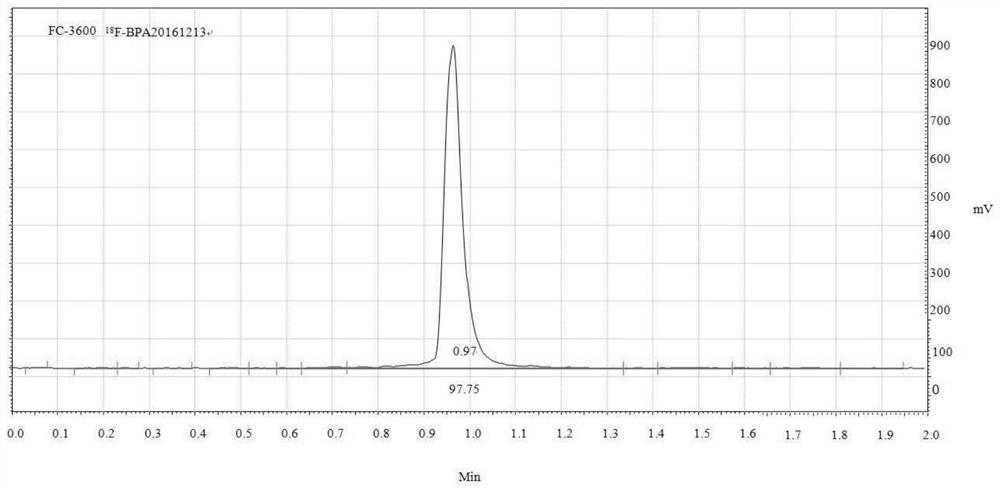
[0133] This example involves 18 Nucleophilic fluorination synthesis of F-BPA, the synthesis process includes the following steps:
[0134] (1) Synthesis of compound 2
[0135] Add 0.84g compound 1, 0.82g dimethylammonium hydrochloride, 0.83g K to the three-necked flask 2 CO 3 , add a reaction solvent composed of 20 mL of dimethyl sulfoxide and 8 mL of water, reflux for 2 h, and add 0.55 g of K to the side port. 2 CO 3 , Continue to reflux for 4h, lower the reaction solution to room temperature, stop the reaction, add 40 mL of saturated K to the reaction solution 2 CO 3 In the aqueous solution, the reaction solution was divided into two layers, which were separated out with a separatory funnel with K 2 CO 3 The remaining reaction solution was extracted twice with 30 mL of ether, the ether layers were combined, the ether layer was washed once with 30 mL of water, and dried over anhydrous magnesium sulfate overnight. The anhydrous magnesium sulfate was removed by filtrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com