Method for synthesizing 4-bromo-7-fluoroisoquinoline
A synthetic method, the technology of isoquinoline, applied in the direction of organic chemistry, etc., can solve the problems of complex operation and post-processing steps, inconvenient large-scale production, complex synthetic route, etc., and achieve convenient operation and post-processing, low raw material cost, and Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
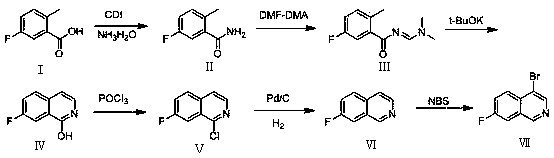
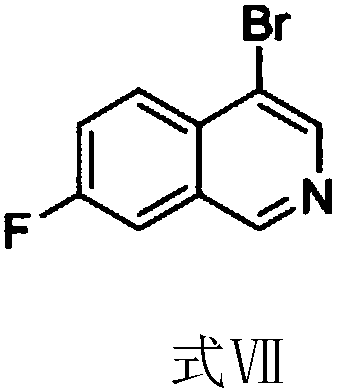
[0038] Such as figure 1 As shown, according to the formula VII provided by the present invention: the synthetic method of 4-bromo-7-fluoroisoquinoline comprises the following steps:
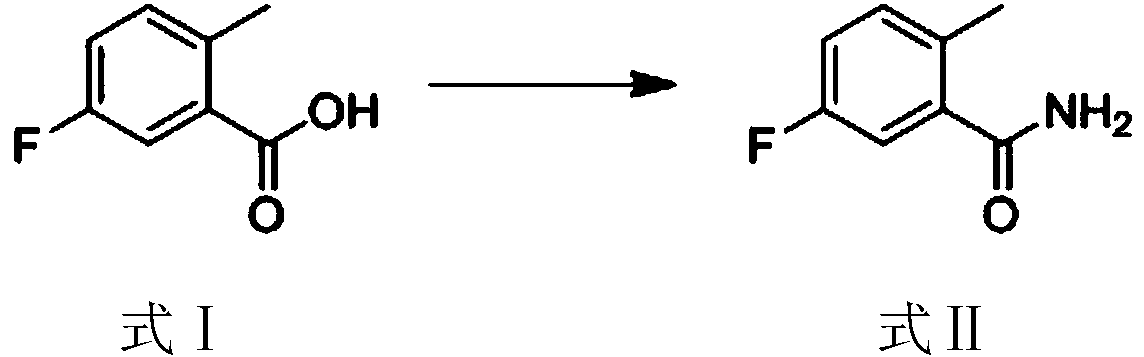
[0039] The synthesis of step (1) 5-fluoro-2-methylbenzamide:
[0040] The raw material 5-fluoro-2-methyl-benzoic acid (formula I) is subjected to carboxylic acid amination reaction to generate 5-fluoro-2-methylbenzamide (formula II);
[0041] Step (2) Synthesis of (E)-N-((dimethylamino)methyl)-5-fluoro-2-methylbenzamide:
[0042] 5-fluoro-2-methylbenzamide (formula II) is subjected to condensation reaction to obtain (E)-N-((dimethylamino)methyl)-5-fluoro-2-methylbenzamide (formula III);
[0043] Synthesis of step (3) 1-hydroxyl-7-fluoroisoquinoline:
[0044] (E)-N-((dimethylamino)methyl)-5-fluoro-2-methylbenzamide (formula III) is subjected to ring closure reaction to obtain 1-hydroxyl-7-fluoroisoquinoline (formula IV);
[0045] Synthesis of step (4) 1-chloro-7-fluoroisoquinoline:
[0046]...
Embodiment 1
[0065] The first step reaction is to use the reactant as 5-fluoro-2-methyl-benzoic acid, the reaction solvent is dichloromethane, and carry out carboxylic acid amination under the action of condensing agent N'N-carbonyldiimidazole (CDI) and ammonia water Reaction, the reaction temperature is 35°C, the reaction time is 3h, and 5-fluoro-2-methylbenzamide is obtained.
[0066] The second step reaction is the condensation reaction of the obtained 5-fluoro-2-methylbenzamide with the reagent N,N-dimethylformamide dimethyl acetal (DMF-DMA), and the reaction solvent is tetrahydrofuran (THF ), the reaction condition was reflux for 2h to obtain (E)-N-((dimethylamino)methyl)-5-fluoro-2-methylbenzamide.
[0067] The third step reaction is to carry out the ring closure reaction of the obtained (E)-N-((dimethylamino)methyl)-5-fluoro-2-methylbenzamide and potassium tert-butoxide (t-BuOK) , the reaction solvent is N,N-dimethylformamide (DMF), and the reaction condition is to react at 120° C....
Embodiment 2
[0072] Synthesis of 5-fluoro-2-methylbenzamide
[0073] Dissolve 5-fluoro-2-methyl-benzoic acid (153g, 0.99mol) in 1500mL of dichloromethane, heat to 35°C, and then add N'N-carbonyldiimidazole (CDI) (193g, 1.2 mol), after reacting for 1 hour, add ammonia water 500mL, react at 35°C for 2 hours, cool to room temperature and separate layers, wherein the aqueous phase is extracted with dichloromethane, then the organic phases are combined, dried and spin-dried to obtain 118g of the product (5 -fluoro-2-methylbenzamide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com