Expression vector of mammalian cell for industrial production
An expression vector and mammalian technology, applied in the fields of molecular biology and biomedicine, can solve the problems of difficulty in the optimization process, failure to screen high-expression cell lines, and high cost, and achieve the effect of avoiding read-through
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 Construction of vector
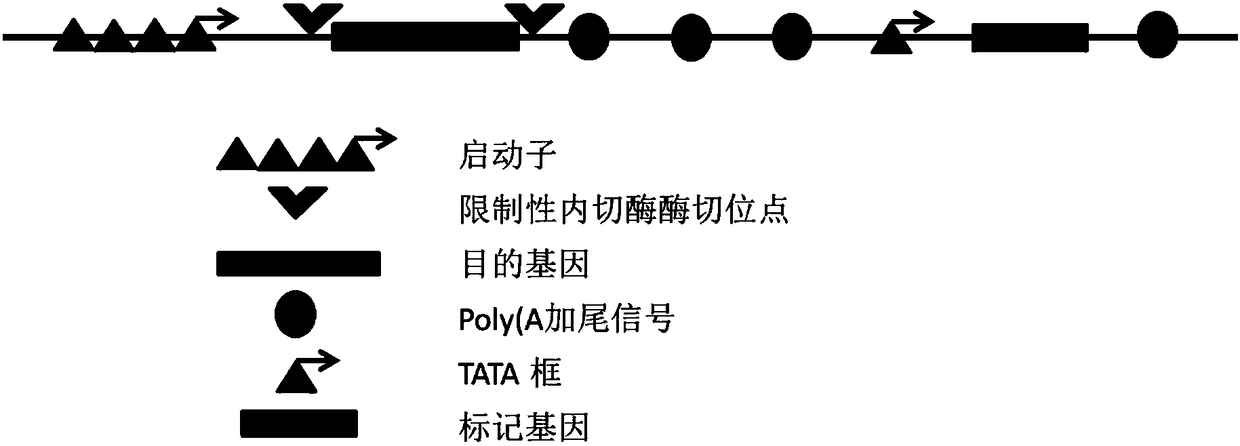
[0041] The sequences of the CMV promoter and its TATA box, mouse DHFR, BGH polyA (BGHpA), beta-globinpolyA (bGpA), TK polyA (TKpA) were obtained through GenBank, and then GAATTC (EcoRI restriction site) was sequenced from 5' to 3' dot)-CMV promoter-Kozak sequence-BamHI and SalI restriction sites-BGHpA-SynpA-bGpA-CMV TATA box-DHFR-TKpA-GAATTC (XhoI restriction site) after the sequence arrangement, artificially synthesized as SEQ The DNA fragment shown in No. 5; EcoRI and XhoI digested and inserted into the pUC19 plasmid, and the plasmid that was successfully inserted into the above DNA fragment after testing is the vector for protein expression in mammalian cells.
Embodiment 2
[0042] Example 2 Expression of GFP (Green Fluorescent Protein).
[0043] 2.1 Construction and expression of the vector
[0044] Four different vectors were constructed with GFP as the target protein: a) pCMV-GFP-SV40-DHFR-TKpA, b) pCMV-GFP-BGHpA-SynpA-bGpA-SV40-DHFR-TKpA, c) pCMV-GFP-BGHpA- TATA-DHFR-TKpA and d) pCMV-GFP-BGHpA-SynpA-bGpA-TATA-DHFR-TKpA, the steps are as follows: refer to GenBank to obtain the sequences of GFP (LN515608.1), SV40 promoter (DM063619.1), and then follow The sequence designed above is artificially synthesized by adding the sequence GAATTC (EcoRI restriction site) at the 5' end and GAATTC (XhoI restriction site) at the 3' end; after EcoRI and XhoI restriction restriction, insert it into the pUC19 plasmid and check The plasmids successfully inserted with the above DNA fragments were transfected into CHO cells (DG44). The sequences of the CMV promoter, CMV promoter TATA box, DHFR, BGHpA, SynpA, bGpA, and TKpA in the vector were as in Example 1.
[0...
Embodiment 3
[0049] Example 3 Expression and activity of anti-human platelet glycoprotein VI single chain antibody (anti-GPVI scFv).
[0050] 3.1 Construction and expression of scFv expression vector
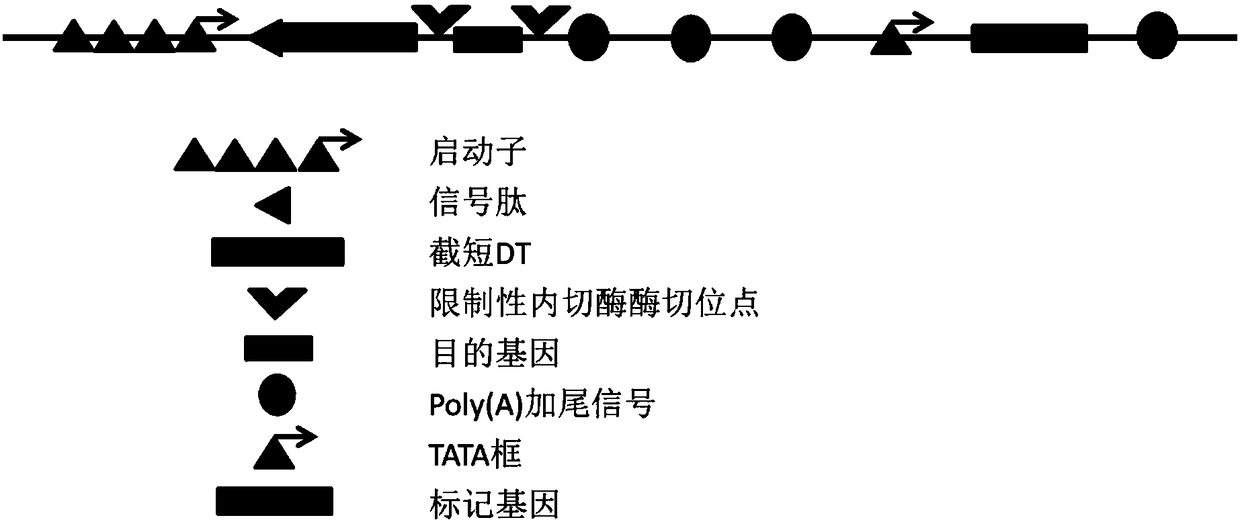
[0051] From the blood of patients with thrombocytopenic purpura (caused by platelet collagen receptor GPVI deficiency) (after transfusion of normal human blood) as a source, nucleated white blood cells (B lymphocytes) with affinity for platelets are isolated, namely The patient's B lymphocytes expressing GPVI antibodies were isolated. Then, using single-cell RT-PCR and specific primers for the heavy chain variable region and light chain variable region of human immunoglobulin IgG, clone the DNA of the light chain variable region and heavy chain variable region of the anti-GPVI antibody sequence, the two are connected by DNA sequence; add Herceptin signal peptide DNA sequence before the light chain variable region sequence; Carry out artificial synthesis, then insert into the vector obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com