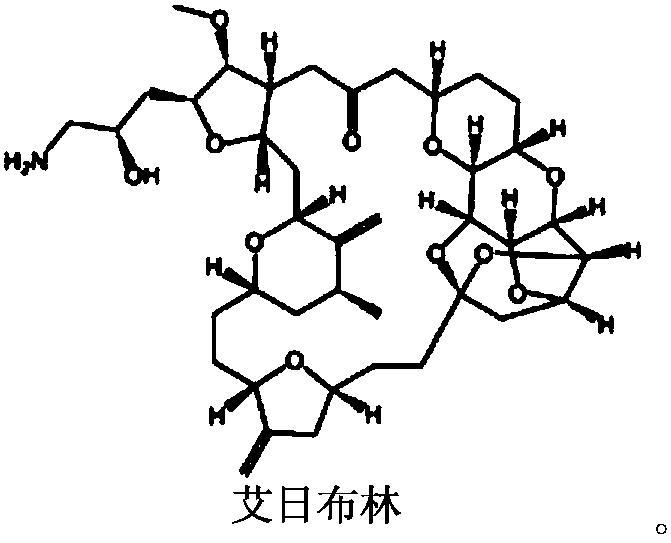
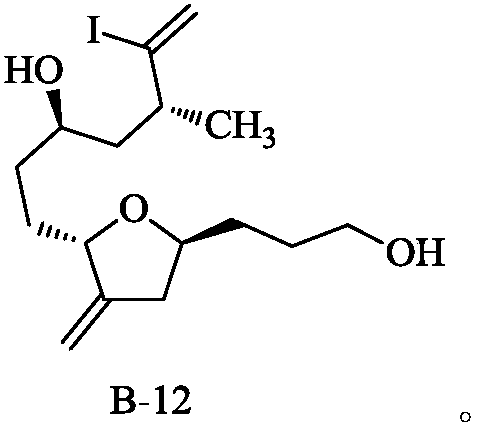
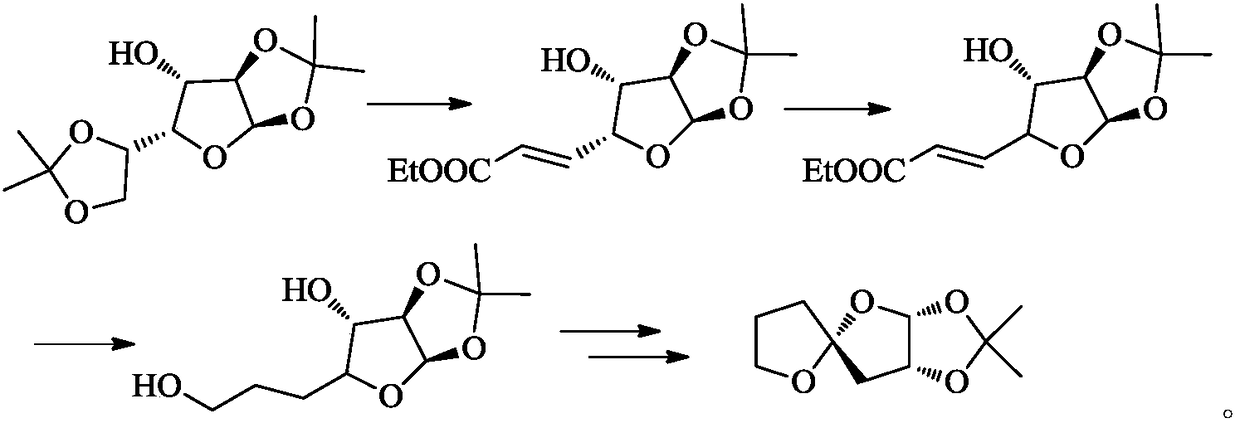
Method for preparing eribulin and intermediate thereof
A technology for compounds and analogs, applied in the field of preparation of eribulin, can solve problems such as difficulty in total synthesis, complex structure, etc., and achieve the effects of avoiding complex and tedious processes and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111]
[0112] Weigh benzylbromoethanol (5.00g) in an eggplant-shaped bottle, dissolve it with acetonitrile (50mL), add triphenylphosphine (9.21g), react at about 80°C for 24h, cool the reaction to room temperature naturally, and evaporate under reduced pressure solvent, added ethyl acetate (50 mL) and stirred for 30 minutes, filtered and washed with ethyl acetate to obtain 9.54 g of white solid with a yield of 86% and a purity of 96%.
Embodiment 2
[0114]
[0115] Weigh quaternary phosphorus salt 9aa (4.76g) in an eggplant-shaped bottle, add anhydrous tetrahydrofuran (300mL), put the reaction solution into dry ice-ethanol to cool, add 2.5M n-BuLi (4mL) dropwise to the reaction, After the dropwise addition, keep the temperature for reaction for 30min, then add 1mL of THF solution of 2d compound (1.32g) dropwise, stir for 10min, then naturally warm up to room temperature, continue the reaction for 1h, add water (50mL) to quench the reaction, and depressurize The solvent was evaporated, ethyl acetate (200 mL) was added, washed with water and saturated sodium chloride successively, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. After separation and purification by column chromatography, 1.80 g of oily compound 4aa was obtained with a yield of 85%.
[0116] Add methanol 10mL, palladium carbon (0.33g) in reaction bottle, add compound C (1.80g), replace hydrogen three ti...
Embodiment 3
[0119]
[0120] Weigh compound E (10.37g) and formylmethylenetriphenylphosphine (12.21g) in an eggplant-shaped bottle, add THF (15ml) to dissolve, react at room temperature for 3h, add tetrabutylammonium fluoride triphenyl Hydrate (36.89g). After reacting at about 50°C for 6 hours, the reaction solution was naturally cooled, concentrated, and separated and purified by silica gel column chromatography to obtain 4.66 g of oily compound G with a yield of 61% and a purity of 95%.
[0121] Add ethanol 100mL, Raney nickel (1.66g) in reaction bottle, add compound G (3.32g), replace hydrogen three times, in H 2 (40Psi) and reacted for 24h, filtered through diatomaceous earth, evaporated the solvent under reduced pressure, separated and purified through silica gel column to obtain 2.94g oily compound D, yield: 87%, purity: 97% d.e.>99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com