Zirconium boride and method for producing same
A technology of zirconium boride and zirconia, applied in the field of combustible zirconium boride and its preparation, can solve the problems of poor material stability and less heat, and achieve the effect of excellent material stability and increased heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] 2. Preparation method of zirconium boride
[0049] The preparation method of the zirconium boride of the present invention has the following steps 1-4.
[0050] (1) Step 1: mixing zirconia, boria and carbon so that the mass ratio of boron oxide to zirconia in the mixture is 90-120% by mass, and the mass ratio of carbon to zirconia is 40-60% by mass;
[0051] (2) Step 2: firing the mixture obtained in step 1 at 400°C to 600°C;
[0052] (3) Step 3: Melting the sintered granules obtained in the step 2 at a temperature above the melting point of zirconium boride through an argon plasma arc in a reducing atmosphere formed by argon;
[0053] (4) Step 4: Slowly cooling the melt obtained in Step 3 to obtain an ingot of zirconium boride.
[0054] Since easily synthesized zirconia and inexpensively obtained boron oxide and carbon are used as raw materials, the method for producing zirconium boride of the present invention can be produced at low cost and can obtain zirconium b...
Embodiment 1)
[0101] For zirconia, powder with a purity of 99.5% by mass and an average particle size of 3 μm (manufactured by Daiichi Zenen Chemical Industry Co., Ltd.), fine powder SGP (manufactured by SEC Carbon Co., Ltd.) for carbon, and B made by BORAX for boron oxide 2 o 3 of powder.
[0102] First, 3.8 kg of zirconia powder, 1.9 kg of carbon powder, and 4.3 kg of boron oxide powder were weighed separately, and they were mixed for 30 minutes with a V-type mixer.
[0103] Thereafter, the obtained mixture was calcined at 480° C. for 3 hours to obtain a sintered granulated product.
[0104] Next, the obtained sintered granules were spread over the electric melting furnace, and electricity was applied while flowing argon gas for generating argon plasma at 0.03 MPa. Electricity is applied at 100 kwh for 1.5 hours to melt at or above the melting point of zirconium boride (unit power consumption: 15 kwh / kg). After the melting was completed, the inflow of argon gas was stopped, a carbon co...
Embodiment 2)
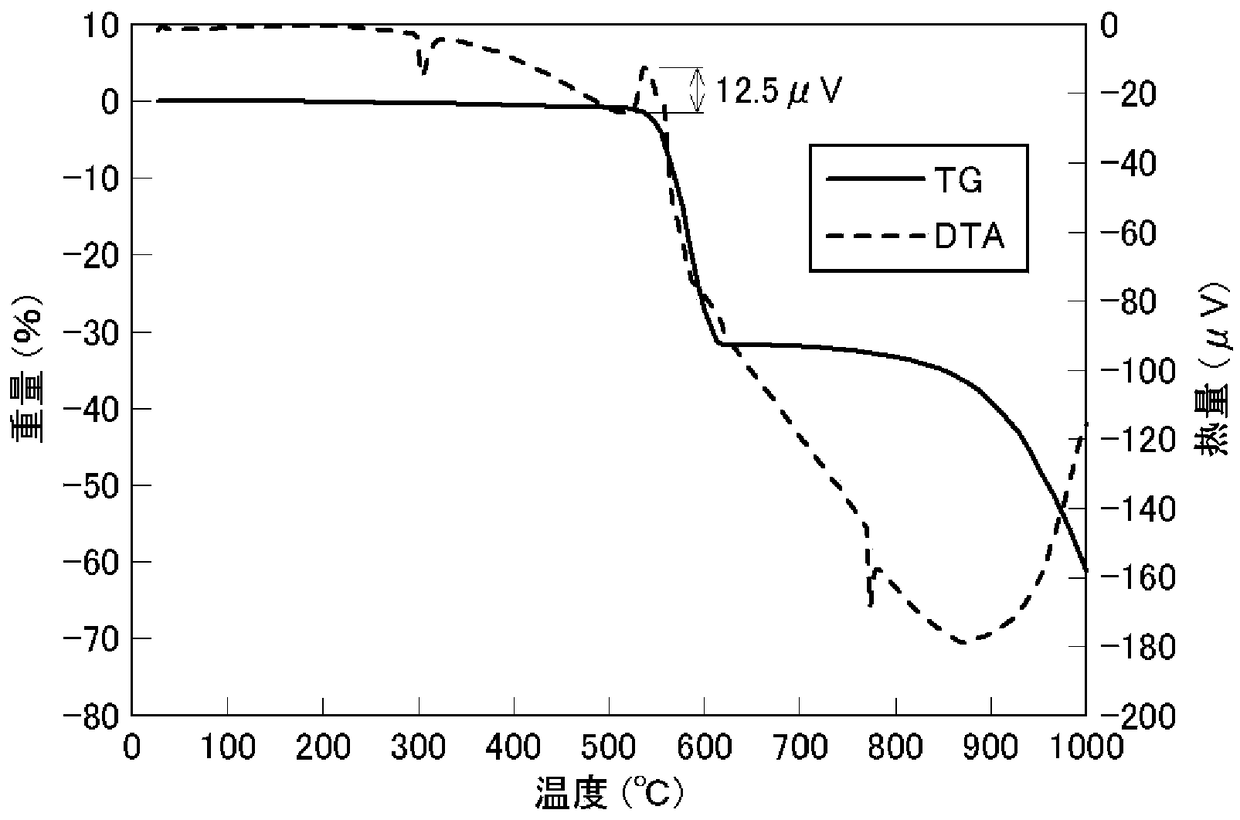
[0109] The ingot obtained in Example 1 was further pulverized with a planetary mill to obtain a powder with an average particle diameter of 1.0 μm. Carbon content is 5.8%, S.A. is 20.6 m 2 / g.
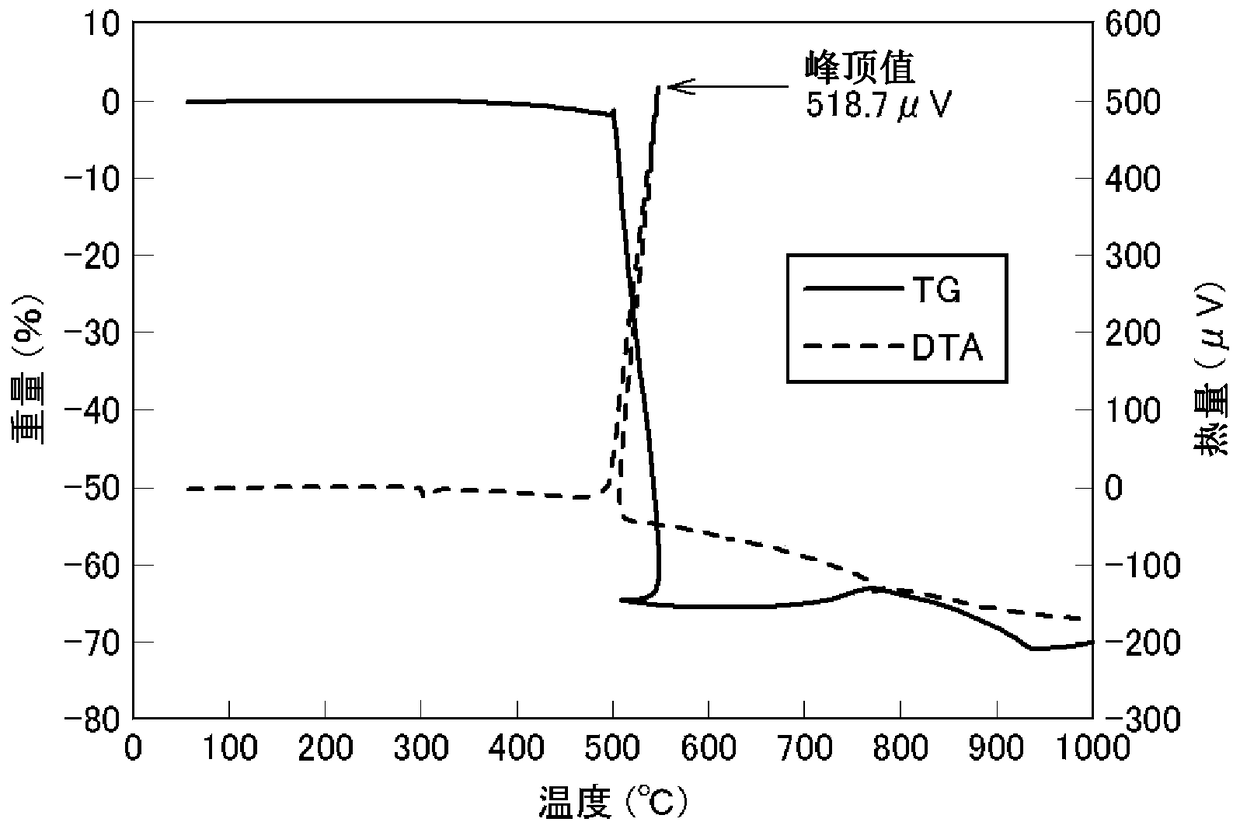
[0110] Potassium perchlorate was added to the obtained zirconium boride powder at a ratio of 40%, mixed well, and 8.1 mg of the obtained mixed powder was subjected to DTA measurement at a temperature increase of 5° C. / m.
[0111] As a result, in the TG-DTA curve, the exothermic peak appeared near 500~550℃, and the exothermic heat seen from the peak was 315.6 μV. Based on this value, the heat release per unit weight is calculated to be 315.6 / 8.1 = 39.0 μV / mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap