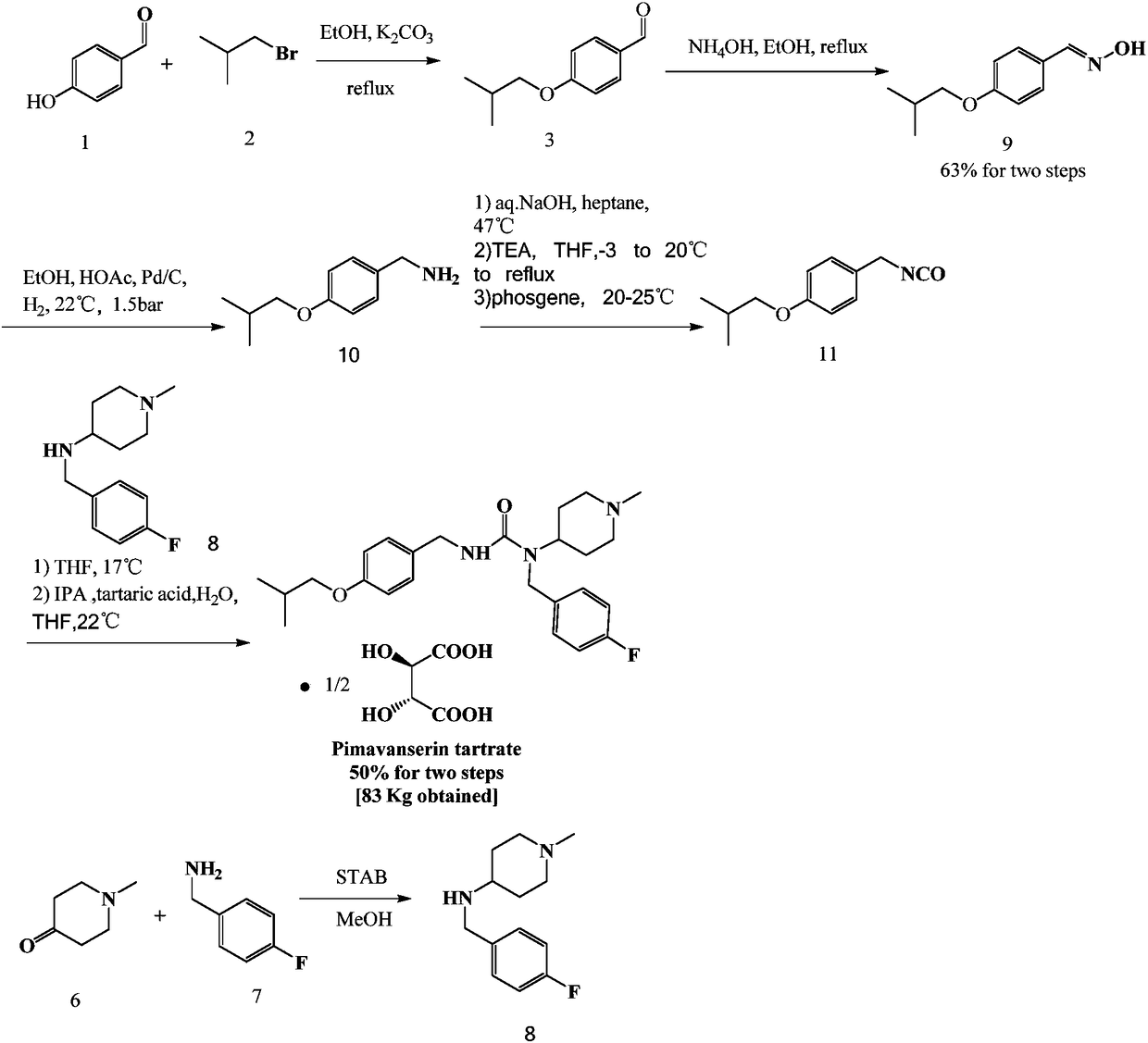
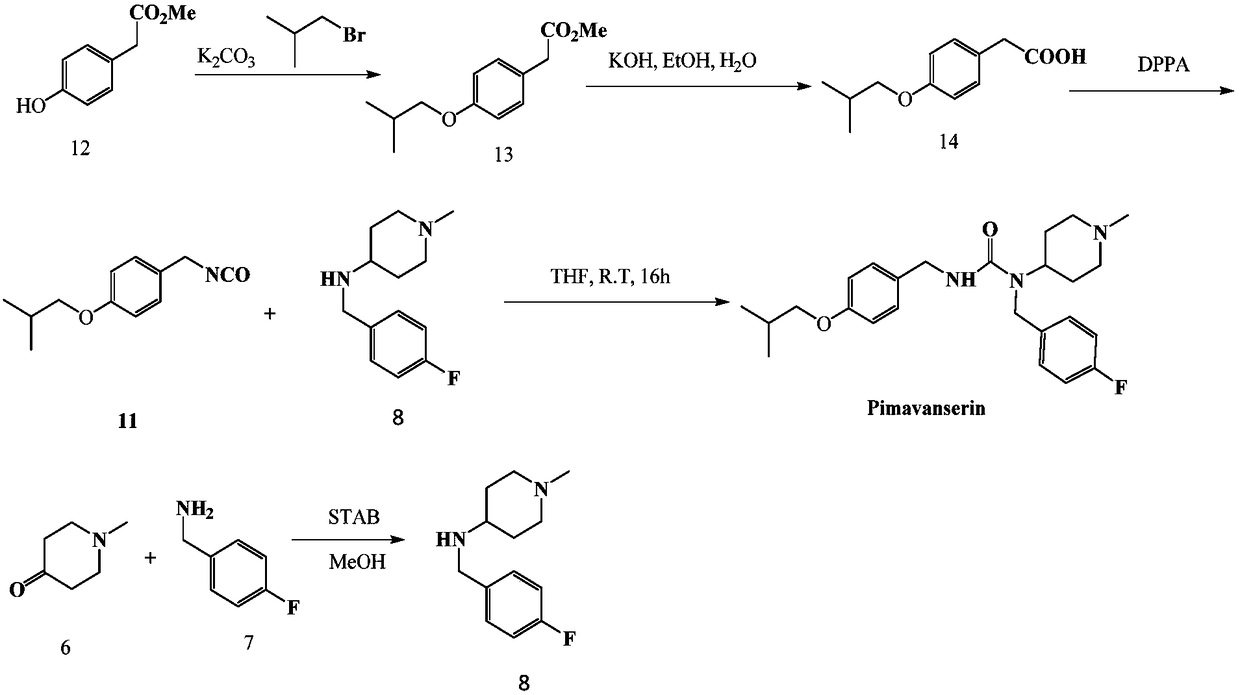
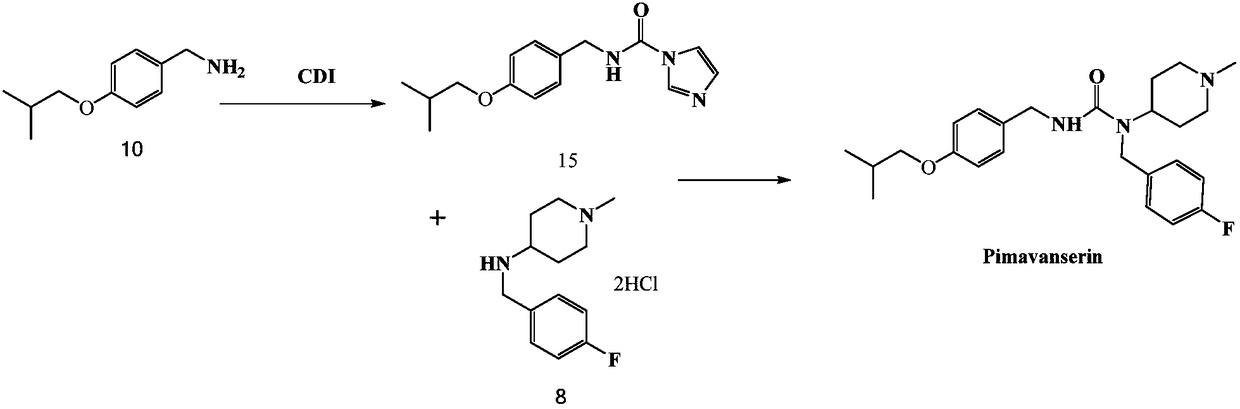
Pimavanserin intermediate and preparation method of pimavanserin
A technology of pimavanserin and intermediates, which is applied in the field of synthesis of pharmaceutical molecules, can solve the problems of large impact on operator's health, unfavorable to industrialized production, high toxicity of chloroformate, etc., and achieves reduction of synthesis cost and easy realization. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of compound 5-a from compound 3 and compound 4-a
[0065]
[0066] Add compound 3 (5g, 28.05mmoL), compound 4-a (2.3g, 30.86mmoL), acetic acid (2.0g, 33.66mmoL), and 30mL dichloromethane into a 100mL three-necked flask. Sodium triacetoxyborohydride (8.9g, 42.1mmoL) was added slowly, and the system reacted at room temperature for 12h. Add 30mL 10% NaOH solution dropwise to the system, stir and separate the liquids, extract the aqueous phase twice with dichloromethane (30mL*2), combine the organic phases, wash once with 30mL saturated NaCl solution, dry over anhydrous sodium sulfate, filter, and the filtrate Concentration gave an off-white solid, which was recrystallized through ethyl acetate / n-heptane = 1 / 10 to obtain compound 5-a, a white solid, 5.73 g, with a yield of 86%.
[0067] MS (m / z): [M+H] + =228.3; Compound 5-a NMR data is as follows: 'H NMR (400MHz, CDCl 3 )(ppm):7.82(d,2H),7.13(d,2H),6.85(s,1H),4.26(s,2H),4.01(d,2H),3.74(s,3H),2.10-1.95 ...
Embodiment 2
[0073] Preparation of compound 5-b from compound 3 and compound 4-b
[0074]
[0075] Add compound 3 (5g, 28.05mmoL), compound 4-b (2.75g, 30.86mmoL), acetic acid (2.0g, 33.66mmoL), and 30mL dichloromethane into a 100mL three-necked flask. Sodium triacetoxyborohydride (8.9g, 42.1mmoL) was added slowly, and the system reacted at room temperature for 12h. Add 30mL 10% NaOH solution dropwise to the system, stir and separate the liquids, extract the aqueous phase twice with dichloromethane (30mL*2), combine the organic phases, wash once with 30mL saturated NaCl solution, dry over anhydrous sodium sulfate, filter, and the filtrate Concentration gave an off-white solid, which was recrystallized through ethyl acetate / n-heptane = 1 / 10 to obtain compound 5-b, a white solid, 6.2 g, with a yield of 88%.
[0076] MS (m / z): [M+H] + =252.3; The NMR data of compound 5-b are as follows: 'H NMR (400MHz, CDCl 3 )(ppm):7.88(d,2H),7.11(d,2H),6.89(s,1H),4.23(s,2H),3.92-3.79(m,4H),3.74(s,3H...
Embodiment 3
[0081] Preparation of compound 5-c from compound 3 and compound 4-c
[0082]
[0083] Add compound 3 (5g, 28.05mmoL), compound 4-c (4.2g, 30.86mmoL), acetic acid (2.0g, 33.66mmoL), 30mL dichloromethane into a 100mL three-necked flask, cool the system to 0°C, Sodium triacetoxyborohydride (8.9g, 42.1mmoL) was added slowly, and the system reacted at room temperature for 12h. Add 30mL 10% NaOH solution dropwise to the system, stir and separate the liquids, extract the aqueous phase twice with dichloromethane (30mL*2), combine the organic phases, wash once with 30mL saturated NaCl solution, dry over anhydrous sodium sulfate, filter, and the filtrate Concentration gave an off-white solid, which was recrystallized from ethyl acetate / n-heptane = 1 / 8 to obtain compound 5-c, a white solid, 7.3 g, with a yield of 87%.
[0084] MS (m / z): [M+H] + =300.4; Compound 5-c NMR data are as follows: 'H NMR (400MHz, CDCl 3 )(ppm):7.87-7.75(m,2H),7.45-7.37(m,3H),7.33(d,2H),7.03(d,2H),4.25(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com