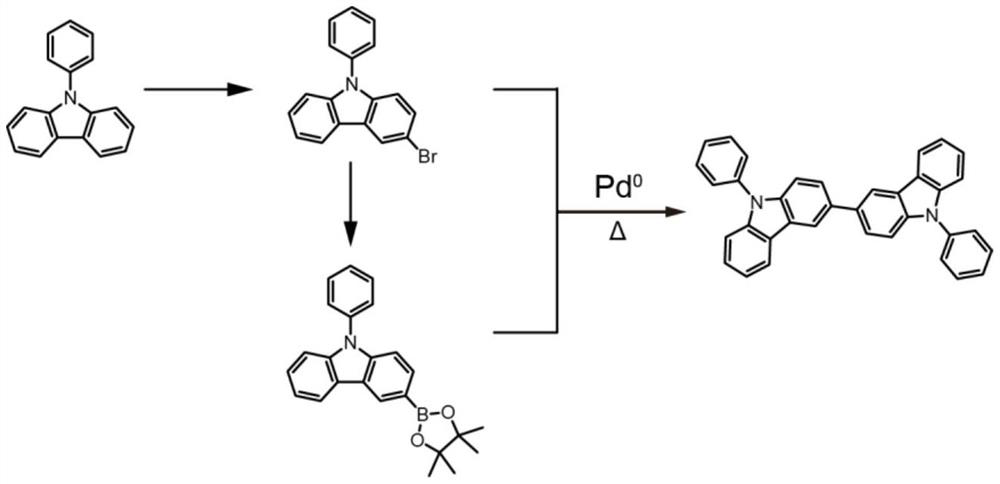
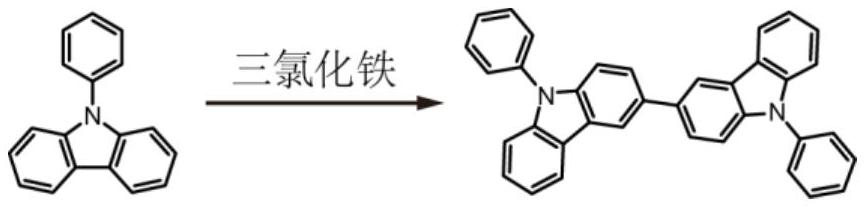
A method for synthesizing 9,9'-diphenyl-3,3'-bicarbazole in one step
A biphenyl and bicarbazole technology, applied in the field of chemical synthesis, can solve the problems of low cost performance and long synthesis process, and achieve the effects of improving conversion rate, simple post-processing, and simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: One-step method for synthesizing 9,9'-diphenyl-3,3'-bicarbazole, the specific method and steps are:
[0032] (1) Under nitrogen atmosphere, place 1.5g (6.2mmol) 9-phenyl-carbazole in a 50mL round-bottomed flask and raise the temperature to 90°C, heat and stir until melting, and within 5 minutes, 0.78mL or 0.31mmol Eaton The reagent was slowly added dropwise, and the solution turned from colorless to dark brown.
[0033] (2) After continuing to react for 12 hours, 10 mL of methanol was added to continue stirring for 2 hours to quench the reaction. The reactant was diluted with dichloromethane and dried with an appropriate amount of magnesium sulfate. Column chromatography was performed using a mixed solvent of petroleum ether and dichloromethane at a volume ratio of 20:1 as an eluent to obtain a white product with a yield of 46%.
[0034] (3) Unreacted raw materials are recovered and reused, and the comprehensive yield of multiple reactions can reach 70%.
Embodiment 2
[0035] Example 2: One-step method for synthesizing 9,9'-diphenyl-3,3'-bicarbazole, the specific method and steps are:
[0036] (1) Under nitrogen atmosphere, place 1.5g (6.2mmol) 9-phenyl-carbazole in a 50mL round-bottomed flask and heat up to 120°C, heat and stir until melting, and within 5 minutes, 0.78mL or 0.31mmol Eaton The reagent was slowly added dropwise, and the solution turned from colorless to dark brown.
[0037] (2) After continuing to react for 12 hours, 10 mL of methanol was added to continue stirring for 2 hours to quench the reaction. The reactant was diluted with dichloromethane and dried with an appropriate amount of magnesium sulfate. Column chromatography was performed using a mixed solvent of petroleum ether and dichloromethane at a volume ratio of 10:1 as an eluent to obtain a white product with a yield of 44%.
[0038] (3) Unreacted raw materials are recovered and reused, and the comprehensive yield of multiple reactions can reach 68%.
Embodiment 3
[0039] Example 3: One-step method for synthesizing 9,9'-diphenyl-3,3'-bicarbazole, the specific method and steps are:
[0040] (1) Under a nitrogen atmosphere, place 1.5g (6.2mmol) 9-phenyl-carbazole in a 50mL round bottom flask and heat up to 110°C, heat and stir until melted, and within 5 minutes, 1.5mL, 0.62mmol Eaton The reagent was slowly added dropwise, and the solution turned from colorless to dark brown.
[0041] (2) After continuing to react for 12 hours, 10 mL of methanol was added to continue stirring for 2 hours to quench the reaction. The reactant was diluted with dichloromethane and dried with an appropriate amount of magnesium sulfate. Column chromatography was performed using a mixed solvent of petroleum ether and dichloromethane at a volume ratio of 3:1 as an eluent to obtain a white product with a yield of 43%.
[0042] (3) Unreacted raw materials are recovered and reused, and the comprehensive yield of multiple reactions can reach 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com