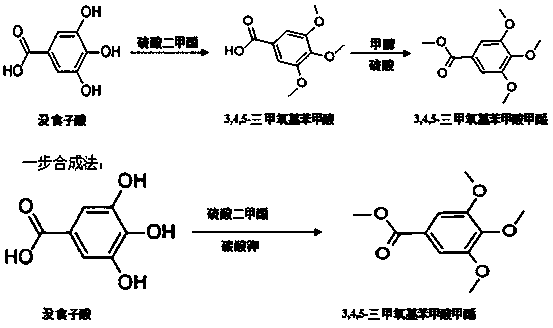
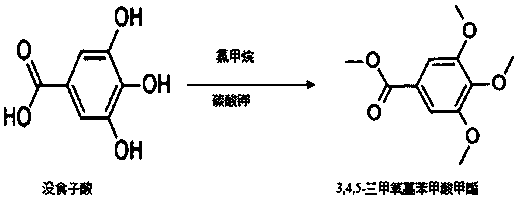
A kind of method for synthesizing methyl 3,4,5-trimethoxybenzoate by one-step method
A technology of methyl trimethoxybenzoate and dimethylformamide, which is applied in the chemical field, can solve the problems of incomplete reaction of raw materials, complicated and lengthy process, and high degree of danger, so as to save time and cost, simplify the preparation process, The effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the method for one-step synthetic 3,4,5-trimethoxymethylbenzoate, concrete method and step are:
[0027] (1) Add 20g of gallic acid, 500ml of DMF, and 20g of potassium carbonate into a four-necked bottle, stir and cool down to below 10°C, pass 50g of chloromethane gas, immediately raise the temperature from 40°C to 110°C within 12 hours , then down to room temperature, concentrated distillation;
[0028] (2) Add 300 mL of water to the concentrated solution, keep stirring, extract three times with ethyl acetate, combine the extracts, concentrate and distill the extracts to obtain a white solid, which is the crude product.
[0029] (3) Put the crude product into 100mL of methanol, stir and heat up to 60-65°C, reflux for 1 hour and then cool down to room temperature. After concentrated distillation, suction filtration and drying, 22.5g of pure product was obtained, the yield was 84.6%, the content was 99.61% by HPLC, and the melting point was 82°C~83°C.
Embodiment 2
[0030] Embodiment 2: the method for one-step synthetic 3,4,5-trimethoxymethylbenzoate, concrete method and step are:
[0031] (1) Add 40g of gallic acid, 1200mL of DMF, and 100g of potassium carbonate into a four-neck bottle, stir and cool down to below 10°C, pass 100g of chloromethane gas, and immediately raise the temperature from 40°C to 110°C within 10 hours , then down to room temperature, concentrated distillation;
[0032] (2) Add 600 mL of water to the concentrated solution, keep stirring, extract three times with ethyl acetate, combine the extracts, concentrate and distill the extracts to obtain a white solid, which is the crude product.
[0033] (3) Put the crude product into 200mL of methanol, stir and heat up to 60-65°C, reflux for 1 hour and then cool down to room temperature. After suction filtration and drying, 45.4g of pure product was obtained, the yield was 85.3%, the content was 99.57% by HPLC, and the melting point was 82°C~83°C.
Embodiment 3
[0034] Embodiment 3: the method for synthesizing 3,4,5-trimethoxymethylbenzoate in one step, concrete method and step are:
[0035] (1) Add 40g of gallic acid, 1500mL of DMF, and 120g of potassium carbonate into a four-neck bottle, stir and cool down to below 10°C, pass 100g of chloromethane gas, and immediately raise the temperature from 40°C to 110°C within 5 hours , then down to room temperature, concentrated distillation;
[0036] (2) Add 600 mL of water to the concentrated solution, keep stirring, extract three times with ethyl acetate, combine the extracts, concentrate and distill the extracts to obtain a white solid, which is the crude product.
[0037] (3) Put the crude product into 200mL of methanol, stir and heat up to 60-65°C, reflux for 1 hour and then cool down to room temperature. After concentrated distillation, suction filtration and drying, 44.8g of pure product was obtained, the yield was 84.2%, the content was 99.88% by HPLC, and the melting point was 83°C~...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com