Preparation method of zinc glycinate chelate
A zinc glycinate and chelate technology, which is applied to the preparation of organic compounds, cyanide reaction preparation, organic chemical methods, etc., can solve the problems of environmental pollution, low absorption and utilization rate, long reaction time, etc., and achieve simple process and high efficiency. The effect of high purity and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Weigh 225.21g (3mol) of glycine, add it to 1250mL water, heat to 80°C, then slowly add 81.39g (1mol) of zinc oxide to the glycine solution, stir until the solution is clear, continue to react for 0.5h, stop the reaction, and the reaction solution After natural cooling, a large amount of zinc glycinate crystals precipitated after 0.5-1h, filtered and dried to obtain 188.2g of zinc glycinate chelate. The reaction yield is 85%, and the molecular formula is C 4 h 8 o 4 N 2 Zn 1 / 2 H 2 O.
example 1
[0014] The zinc glycinate reaction solution in Example 1 was filtered to remove the crystallization product, and the filtrate was set aside. Filtrate is used as reaction solvent and raw material, adds 27g zinc oxide, and heating continues reaction. Recycling, glycine is 100% utilized, and the reaction yield can almost reach 100%. The molecular formula is C 4 h 8 o 4 N 2 Zn 1 / 2 H 2 O.
Embodiment 3
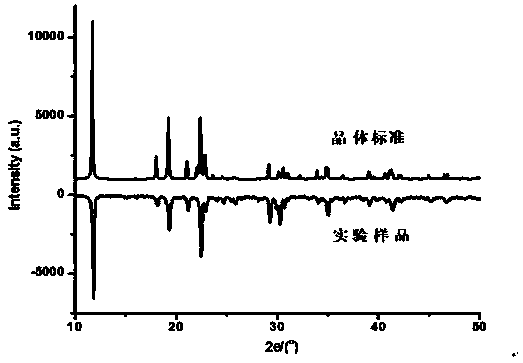
[0016] The zinc glycinate chelate prepared above is cultivated by single crystals to obtain crystals and solve the crystal structure; the product is characterized by X-ray powder diffraction, and its purity is determined by comparing the powder diffraction data of its crystals.
[0017] The X-ray powder diffraction figure of zinc glycine chelate is as attached figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com