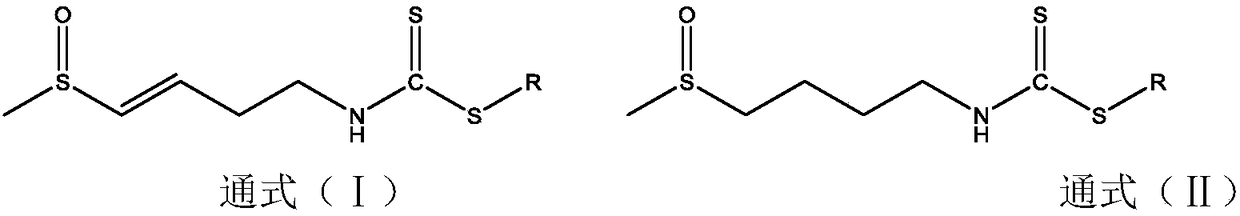
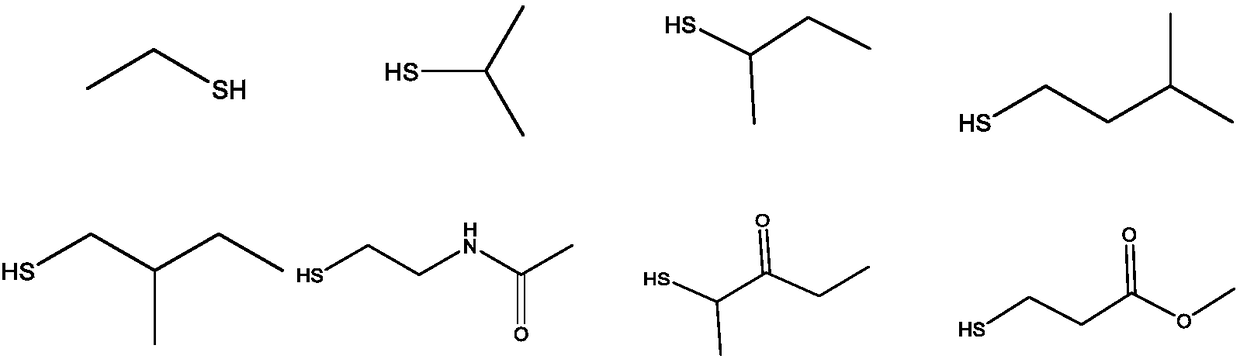
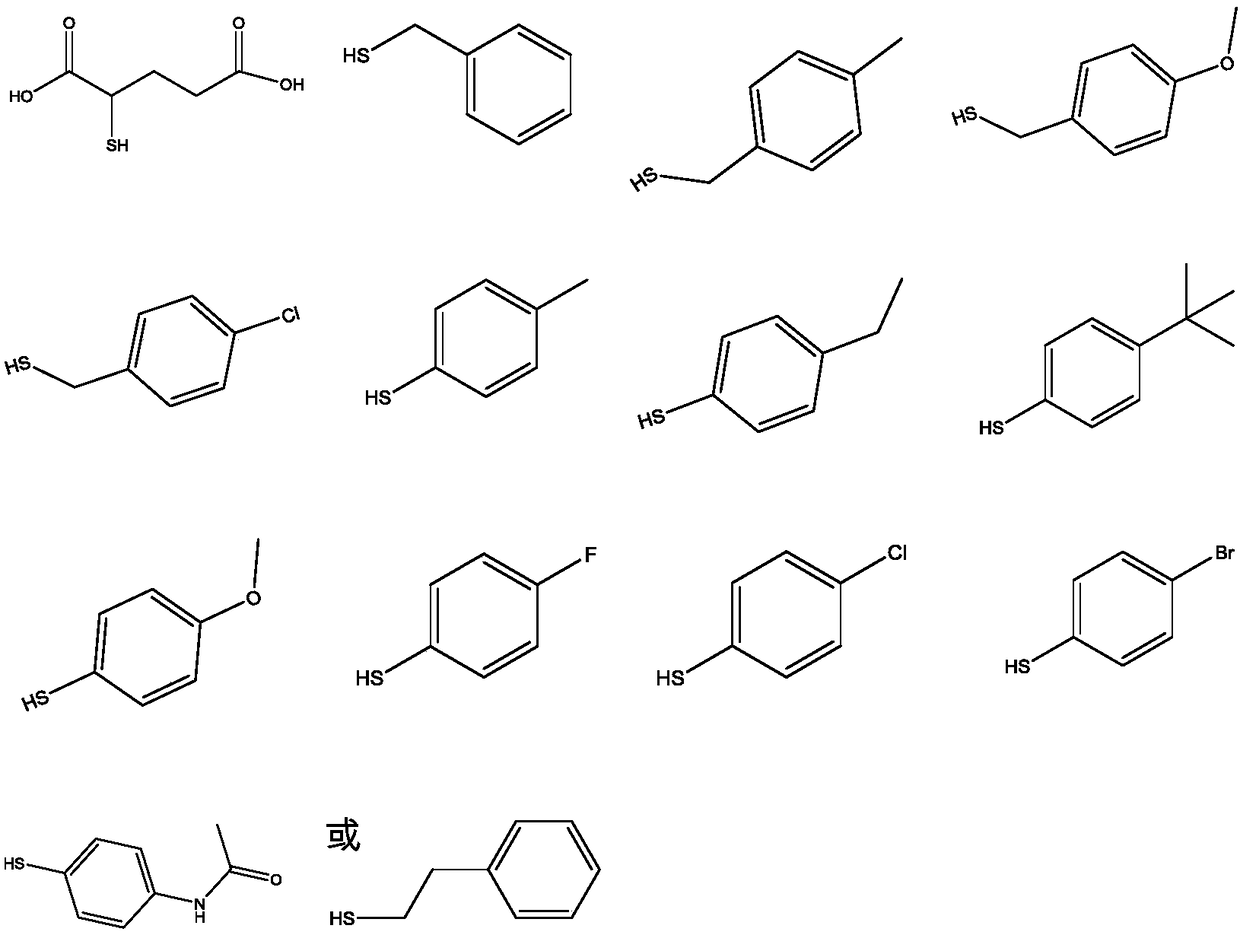
Preparation of dithiocarbamate compound and application of dithiocarbamate compound in drug for inhibiting cancer cell proliferation and/or treating cancer
A compound and cancer cell technology, applied in drug combination, organic chemistry, antineoplastic drugs, etc., can solve problems such as instability of radhamin and sulforaphane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of R101:
[0034] Weigh raw NaHCO 3 Put 0.26g (3.2mmol) in a 100ml single-necked flask, add 10ml of water to dissolve, stir magnetically, then weigh 0.19g (3mmol) of ethanethiol, add it, and finally weigh 0.53g (3mmol) of radishin, dilute and dissolve with 20ml of ethanol and add to the reaction solution During the reaction at room temperature, the reaction time was 5 h, during which the reaction was followed by thin-layer chromatography, and the developing solvent was ethyl acetate: methanol = 5:1. After the reaction was completed, the organic solvent ethanol was evaporated, diluted with 20ml of water, extracted three times with 60ml of ethyl acetate, 20ml each time, and the extract was extracted with anhydrous MgSO 4 After drying and filtering, the filtrate was spin-evaporated to give a yellow oil, and freeze-dried to obtain 0.49 g of the final product, with a yield of 69.3%. C 8 h 16 NOS 3 , MS(ES+)m / z(M+H) + :238.0389; 1 H NMR (400MHz, MeOD-d 4 ...
Embodiment 2
[0036] Preparation of W101:
[0037] Weigh raw NaHCO 3 Put 0.26g (3.2mmol) in a 100ml single-necked flask, add 10ml of water to dissolve, stir magnetically, then weigh 0.19g (3mmol) of ethanethiol, add it, and finally weigh 0.53g (3mmol) of radishin, dilute and dissolve with 20ml of ethanol and add to the reaction solution During the reaction at room temperature, the reaction time was 5 h, during which the reaction was followed by thin-layer chromatography, and the developing solvent was ethyl acetate: methanol = 5:1. After the reaction was completed, the organic solvent ethanol was evaporated, diluted with 20ml of water, extracted three times with 60ml of ethyl acetate, 20ml each time, and the extract was extracted with anhydrous MgSO 4After drying and filtering, the filtrate was spin-evaporated to give a yellow oil, and freeze-dried to obtain 0.63 g of the final product, with a yield of 87.5%. C 8 h 18 NOS 3 , MS(ES+)m / z(M+H) + :240.0545; 1 H NMR (400MHz, DMSO-d 6 )...
Embodiment 3
[0082] (1) Experimental cell lines, human skin malignant melanoma cell line A375, human liver cancer cell SMMC-7721, human lung cancer A549, human colon cancer cell HCT116, human cervical cancer cell Hela, among which A375 and SMMC-7721 were cultured in DMEM medium , HCT116, A549, and Hela were cultured in RPMI-1640 medium and passaged after routine digestion with 0.25% trypsin.
[0083] (2) After digesting the cancer cells in the logarithmic growth phase into single cells, 3×10 4 Seed in 96-well plate at a density of cells / ml, add 90 μL of cell suspension to each well, incubate in a 37°C incubator containing 5% CO2 for 12 hours, add 10 μL of carbamate drugs and control drug radish All the elements acted on the same cell, and three replicate wells were set up for each concentration. After 72 hours, 10 μL of cck-8 solution was added to each well and incubated for 1 hour. The absorbance value at 490 nm was detected with a microplate reader and the inhibition rate was calculated....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com