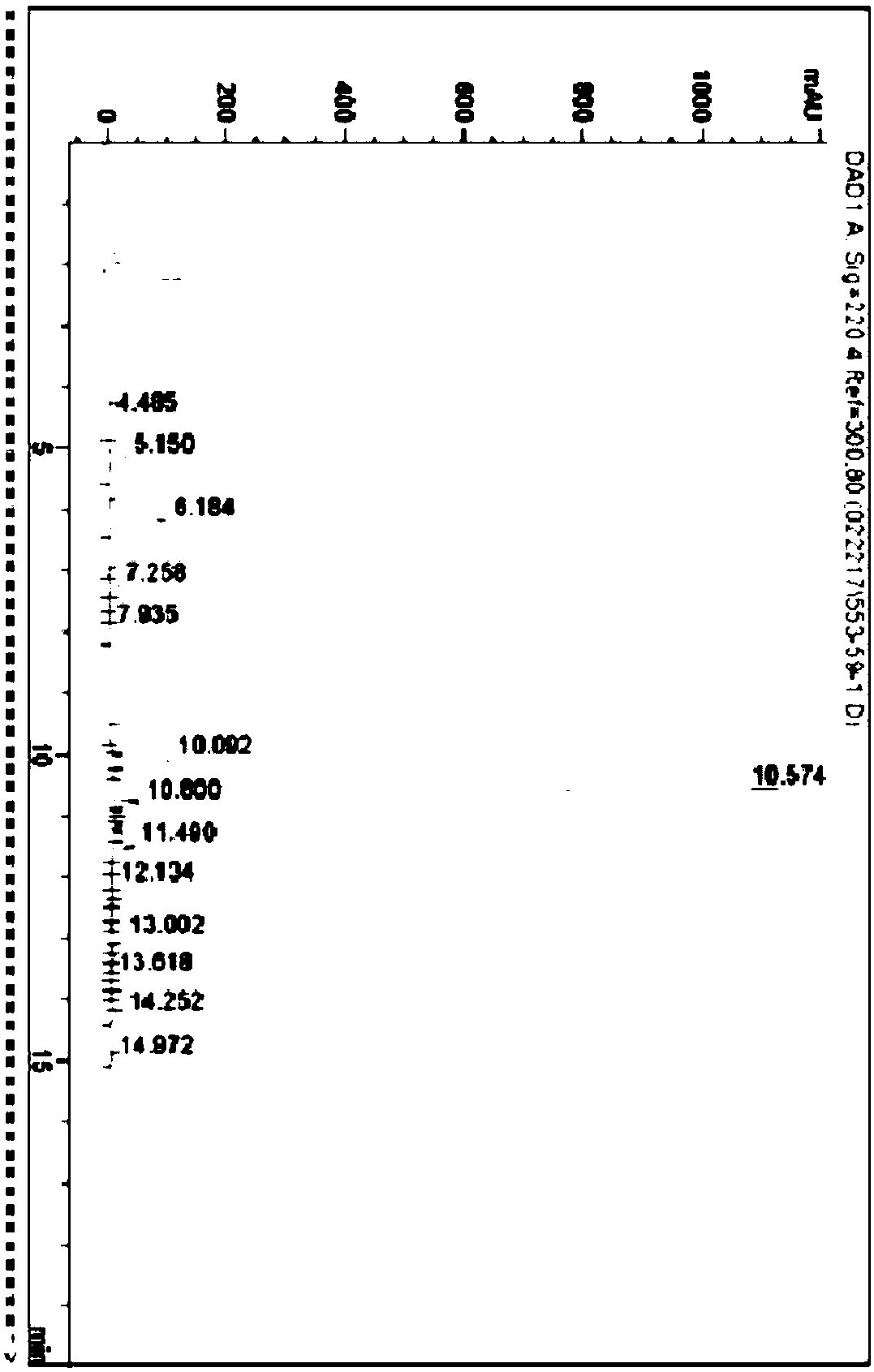
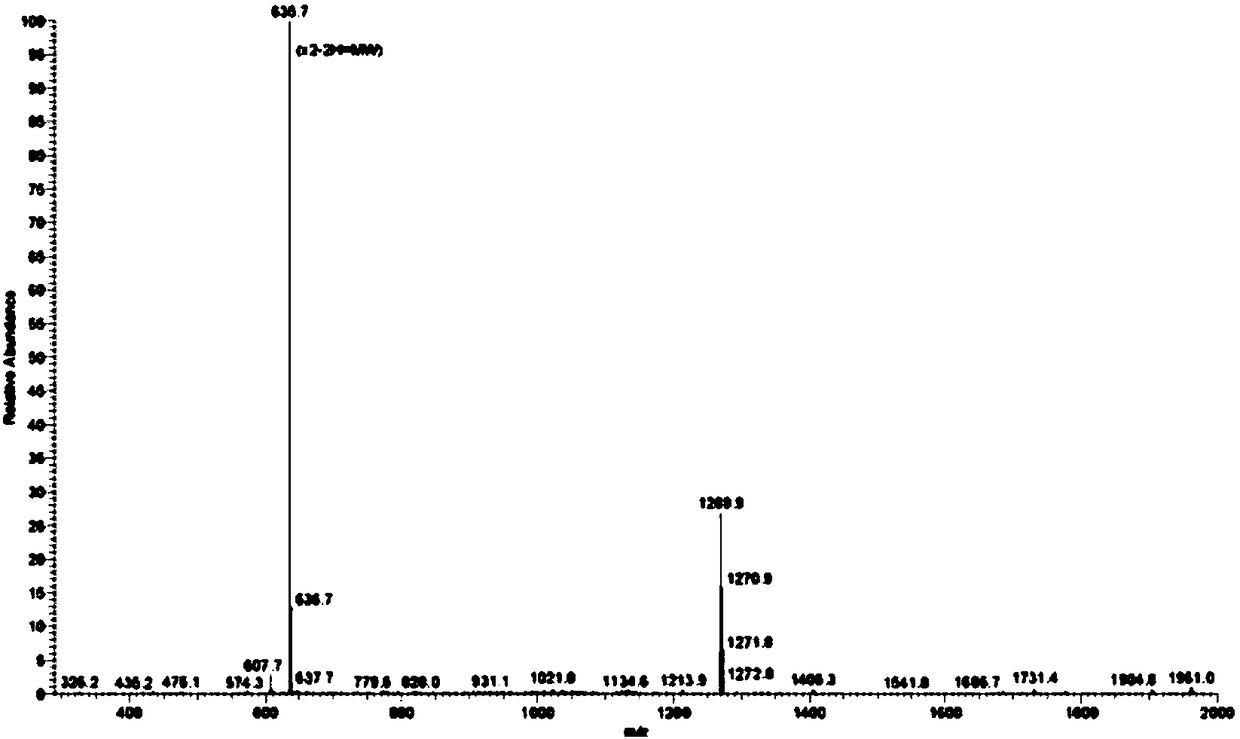
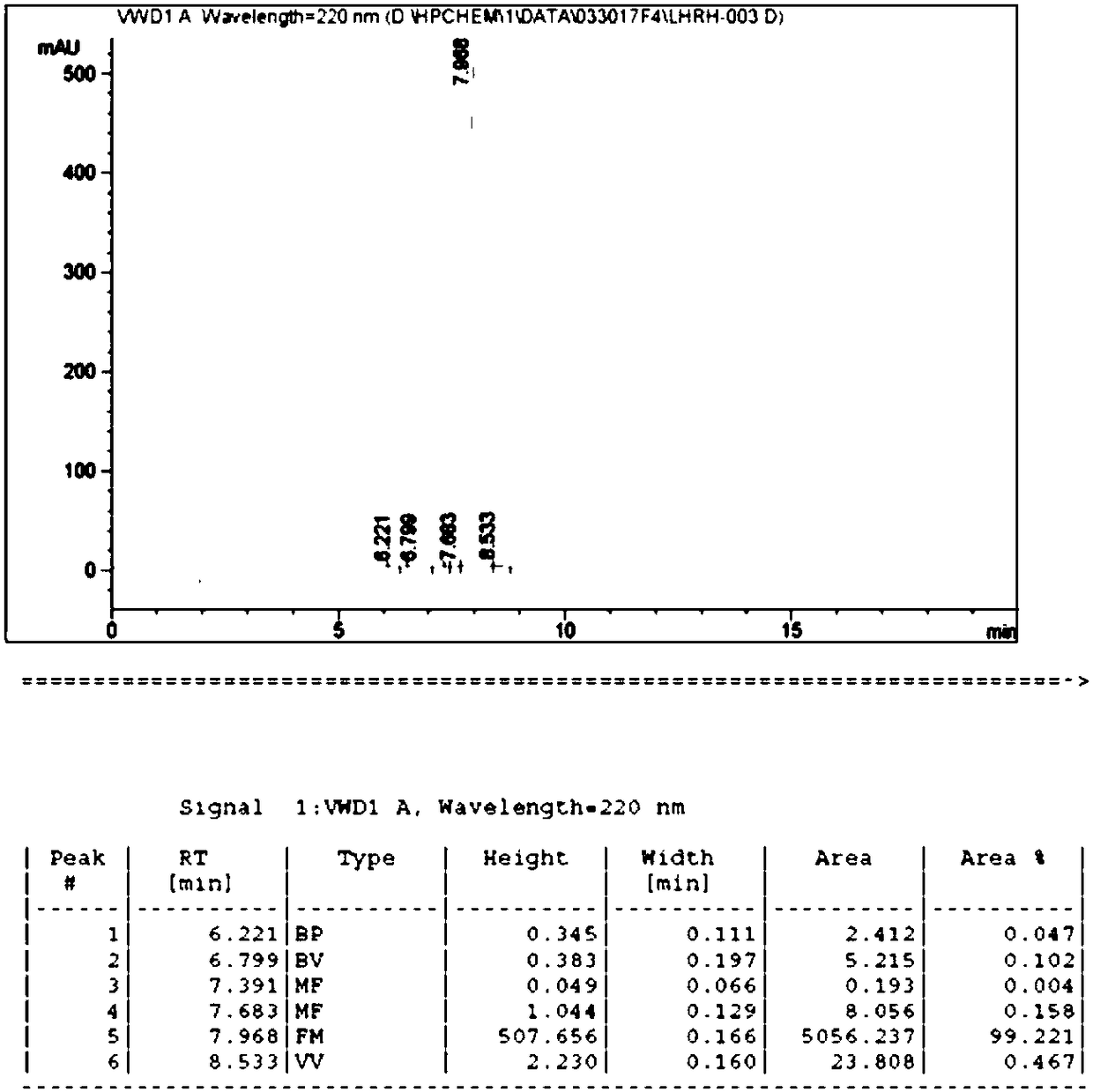
Method for synthesizing goserelin with fragment method
A synthetic method, the technology of goserelin, which is applied in the field of synthesis of goserelin by the fragment method, can solve the problems affecting the separation of the final product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of H-Arg(pbf)-Pro-Azagly-Resin.
[0038] 741 g of MBHA Resin with a degree of substitution of 0.54 mmol / g was added to the solid phase reactor, DCM was added to swell the resin for 2 hours, DCM was removed, and DMF washed once. Add 5% TEA / DMF (v:v) mixed solution of 2.5 times the volume of the resin layer to neutralize the resin for 5 minutes, remove the reaction solution, wash twice with DMF, twice with methanol, twice with DMF, and the ninhydrin method Tested positive.
[0039] Dissolve 648g Rink Amide Linker and 432g HBTU in DMF under ice bath conditions, add to the resin and react for 5 minutes, then add 264mL NMM, and react at room temperature for 2.5 hours. The end point of the reaction is determined by the ninhydrin method. After the reaction, DMF was washed twice, methanol was washed once, and DMF was washed twice.
[0040] 2.5 times the volume of the resin layer was added with DBLK to remove the Fmoc protection. After 30 minutes of reacti...
Embodiment 2
[0045] Example 2: H-Arg-Pro-Azagly-NH 2 preparation.
[0046] Prepare 10L of cleavage reagent, including 9.5L of TFA, 0.25L of water, and 0.25L of triisopropylsilane, and put it in a -20°C refrigerator to pre-cool for more than 30 minutes. Weigh 1000 g of the peptide resin obtained by the method described in Example 1, slowly add the resin to 10 L of cleavage reagent under stirring conditions, and react at room temperature for 3 hours. Filter the resin, collect the filtrate, slowly add the filtrate to 100L of cold ether for precipitation, centrifuge, wash three times with cold ether, and dry under reduced pressure to obtain crude peptide H-Arg-Pro-Azagly-NH 2 178g.
Embodiment 3
[0047] Example 3: H-Arg-Pro-Azagly-NH 2 preparation.
[0048] 12.6 g of Fmoc-Pro-OH was dissolved in 100 mL of DMF solvent, and 8.6 g of EDC·HCl, 6.0 g of HOBt, and 12.46 mL of NMM were added under ice-water bath conditions. After the weight is completely dissolved, slowly add 5.0 g of H-Azagly-NH 2 HCl, the reaction mixture continued to react at 25°C. The progress of the reaction was monitored by TLC on a thin-layer chromatography plate, and after the reaction was completed, it was neutralized with 600 mL of a 5% aqueous phosphoric acid solution. The aqueous phase was extracted with 400 mL of DCM, and the DCM organic phase was washed with 200 mL of water and 200 mL of saturated brine, and dried over anhydrous sodium sulfate after the washing was completed. Filtration, rotary evaporation under reduced pressure to remove DCM to obtain a solid, the obtained solid was vacuum-dried and weighed 20.0 g Fmoc-Pro-Azagly-NH 2 Crude product, purity 66%.
[0049] To 20.0g Fmoc-Pro-A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com