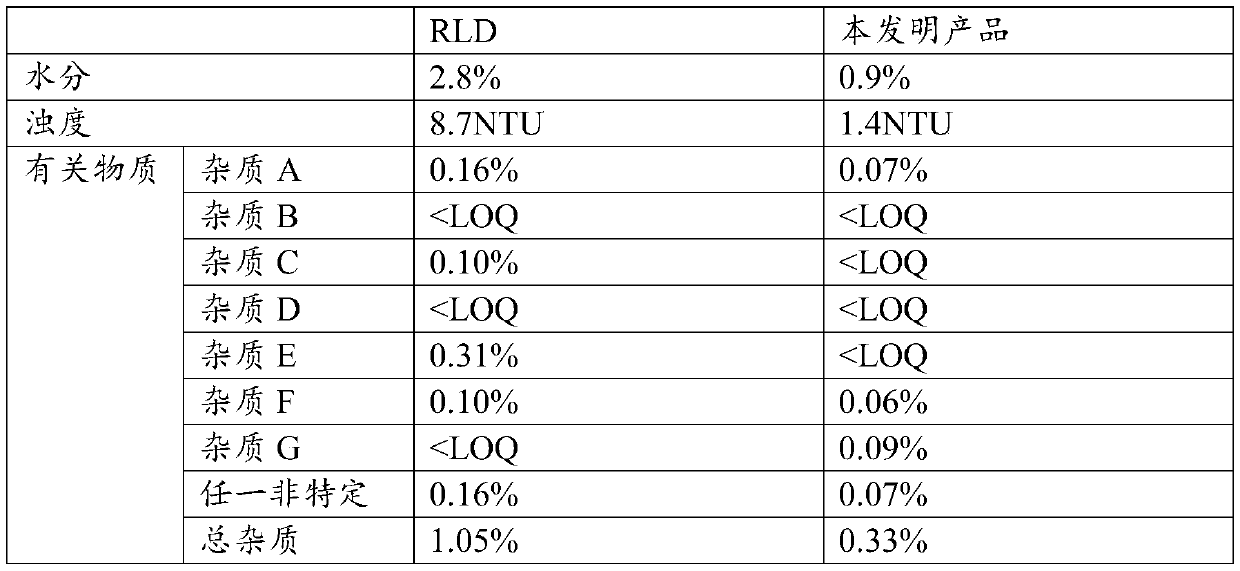
A kind of production process of calcium levofolinate freeze-dried powder for injection
A technology of calcium levofolinate and freeze-dried powder injection, applied in the field of medicine, can solve the problems of re-contaminated product content, low content of liquid medicine, difficult to control, etc., so as to shorten the time of liquid preparation process, low moisture content, and ensure safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A production process of calcium levofolinate freeze-dried powder for injection, comprising the following steps:
[0032] (1) Add water for injection at 40°C in the liquid mixing tank;
[0033] (2) By mass, add 1 part of mannitol and stir until dissolved, keeping the liquid temperature at 40°C;
[0034] (3) Add 1 part of calcium levofolinate raw material and stir until dissolved, lower the temperature of the liquid medicine to 20°C, adjust the pH to 7.8, add water for injection to dilute to 1000 parts;
[0035] (4) After sterilizing and filtering, filling and freeze-drying, the preparation product is obtained.
Embodiment 2
[0037] A production process of calcium levofolinate freeze-dried powder for injection, comprising the following steps:
[0038] (1) Add water for injection at 50°C in the liquid distribution tank;
[0039] (2) By mass, add 1 part of mannitol and stir until dissolved, keeping the liquid temperature at 50°C;
[0040] (3) Add 1 part of calcium levofolinate raw material and stir until dissolved, lower the temperature of the liquid medicine to 30°C, adjust the pH to 8.2, add water for injection to dilute to 1000 parts;
[0041] (4) After sterilizing and filtering, filling and freeze-drying, the preparation product is obtained.
[0042] The endotoxin of the bulk drug is less than 0.500 EU / mg, and the endotoxin of mannitol is less than 2.5 EU / g.
[0043] Described (3) step adopts 0.05mol / L sodium hydroxide solution to adjust the pH value.
Embodiment 3
[0045] A production process of calcium levofolinate freeze-dried powder for injection, comprising the following steps:
[0046] (1) Add 90% of the demanded water for injection at 45°C in the liquid distribution tank;
[0047] (2) By mass, add 1 part of mannitol and stir until dissolved, keeping the liquid temperature at 45°C;
[0048] (3) Add 1 part of calcium levofolinate raw material and stir until dissolved, lower the temperature of the liquid medicine to 25°C, adjust the pH to 8.0, add the remaining demanded amount of water for injection and set the volume to 1000 parts;
[0049] (4) After sterilizing and filtering, filling and freeze-drying, the preparation product is obtained.
[0050] The endotoxin of the bulk drug is less than 0.500 EU / mg, and the endotoxin of mannitol is less than 2.5 EU / g.
[0051] Described (3) step adopts 0.05mol / L hydrochloric acid solution to adjust pH value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com