Method for improving fluorescence signal intensity of fluorescent protein
A fluorescent protein and fluorescent signal technology, applied in the biological field, can solve the problem that the three-dimensional structure is not easy to form correctly, and achieve the effect of improving the intensity of the fluorescent signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Construction of fusion fluorescent protein IGFP
[0032] This example exemplifies a fusion fluorescent protein IGFP spliced with the N-terminal signal peptide of the naturally occurring protein Cry1Ia and the green fluorescent protein GFP, and its amino acid sequence is SEQ ID NO:11.
[0033] The characteristics of the IGFP protein are: the N-terminal signal peptide sequence of Cry1Ia is located at the N-terminal of the green fluorescent protein GFP; there are no other redundant amino acids between the N-terminal signal peptide of Cry1Ia and the amino acid sequence of the green fluorescent protein GFP; the C-terminal of IGFP contains 6 A histidine, used to extract IGFP protein if necessary. This histidine tag sequence is not required to construct the fusion fluorescent protein. The coding polynucleotide sequence of the IGFP protein can be obtained from at least three ways: 1) by using the polynucleotide sequence of the N-terminal signal peptide of coding C...
Embodiment 2
[0034] Example 2: Construction of prokaryotic cell expression vector pAcIGFP fused with fluorescent protein IGFP
[0035] This example exemplifies a prokaryotic cell expression vector for the fusion fluorescent protein described herein, which can be used to monitor the expression pattern of the promoter Pac of the polynucleotide chain encoding the Cry1Ac protein (ie, the promoter of the cry1Ac gene).
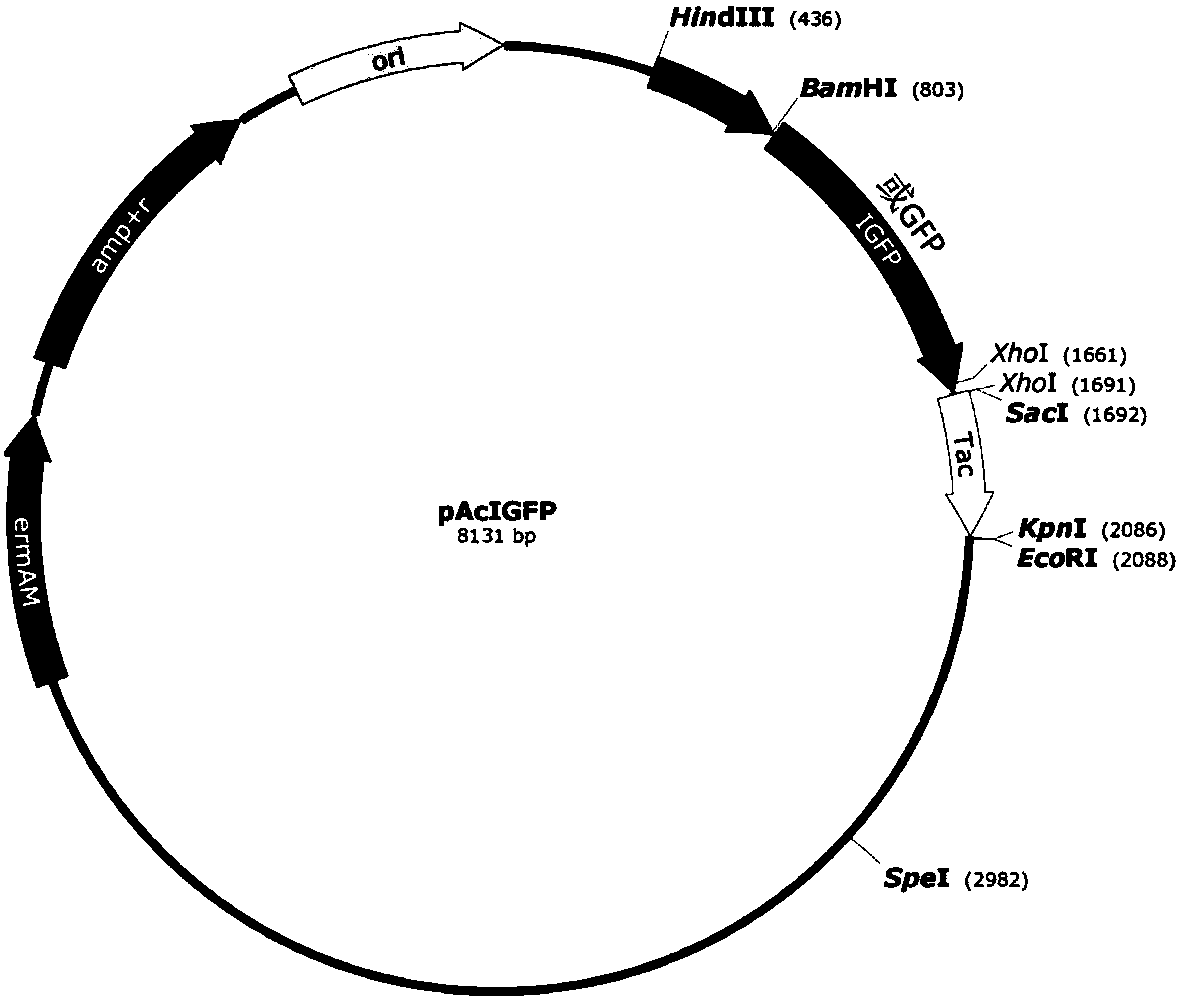
[0036] The 5' of the polynucleotide chain encoding the IGFP protein is connected to the promoter Pac of the cry1Ac gene, and the 3' is connected to the terminator Tac of the cry1Ac gene to form a complete expression frame, and this expression frame is inserted into a deletion restriction The pHT304 vector (Olivia Arantes and Didier Lereclus, Gene, 1990) of the Dicer SacI recognition site——p304ΔSacI, forming the pAcIGFP expression vector ( figure 1 ). As a control, the coding polynucleotide sequence of green fluorescent protein (with 6 histidines at the C-terminal) was also conn...
Embodiment 3
[0038] Example 3: Application of expression vector pAcIGFP in Bacillus thuringiensis
[0039] The expression vectors pAcIGFP and pAcGFP were respectively transformed into competent cells of Bacillus thuringiensis by electroporation. The screened single clones were shaken and cultured in liquid LB medium (Luria-Bertani medium) containing 25 μg / mL erythromycin (28.5° C., 200 rpm). At 9, 12, 24, 36, 48, 60, and 72 hours after culture, the fluorescence signal intensity of the cells was monitored respectively. The results showed that the fluorescence signal intensity of the Bacillus thuringiensis cells containing the pAcIGFP vector was consistently about four to six times higher than that of the Bacillus thuringiensis cells containing the pAcGFP ( figure 2 A). This result fully demonstrates that, compared with GFP, IGFP is more suitable for use as a fluorescent marker for Bacillus thuringiensis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com