Astragaloside loaded polycaprolactone membrane and preparation method and applications thereof
A technology of polycaprolactone and astragaloside IV, applied in the field of medicine, can solve the problems of long-term clinical application and side effects, achieve good clinical curative effect, promote recovery, and inhibit exudation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
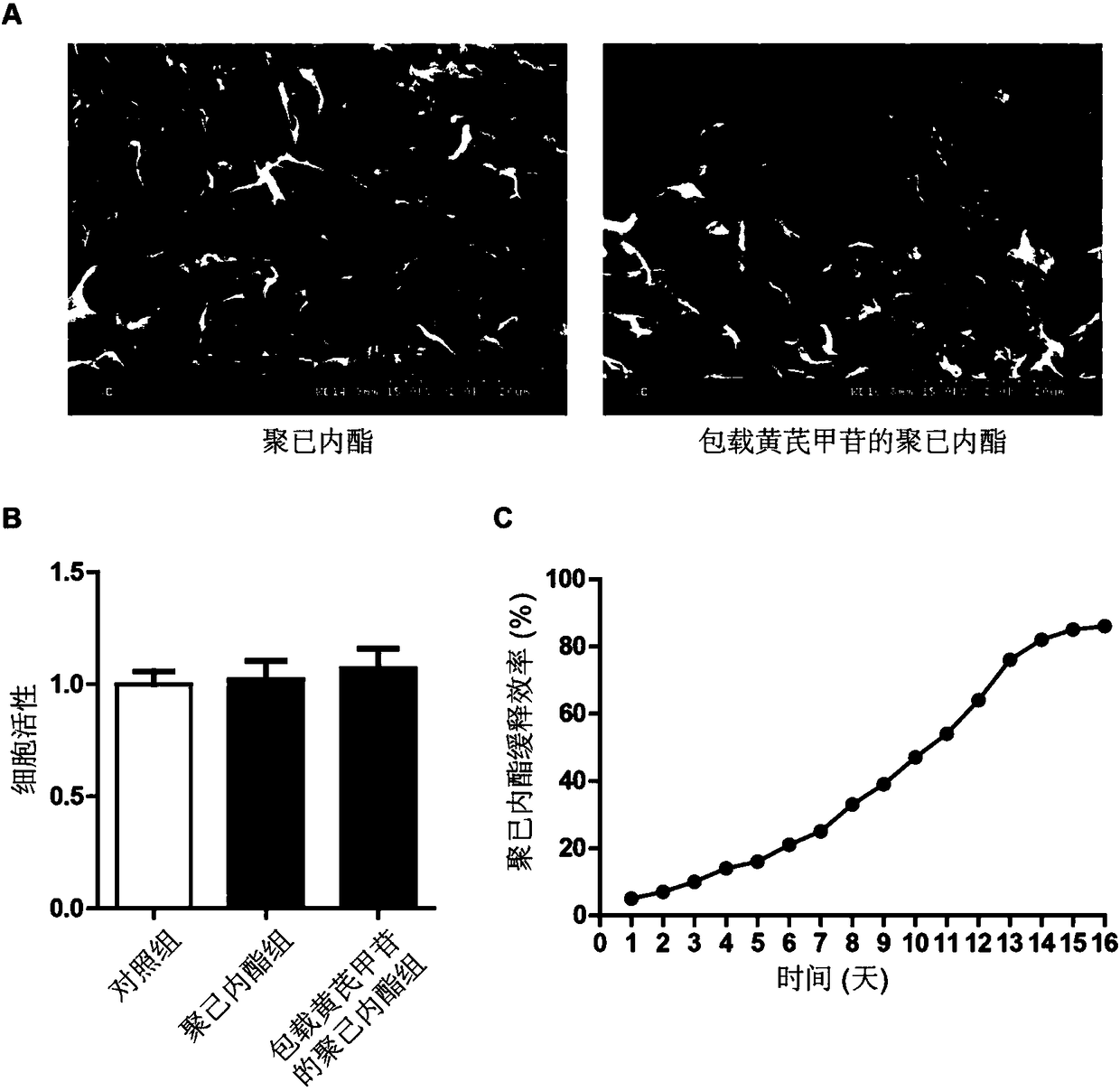
[0058] Preparation and Characterization of Polycaprolactone Films Loaded with Astragaloside IV
[0059] The preparation of polycaprolactone film loaded with astragaloside includes the following steps: (1) Preparation of polycaprolactone solution: polycaprolactone is put into trifluoroethanol solution, stirred overnight at 37 ° C, and prepared into polycaprolactone Caprolactone solution, wherein the weight-to-volume ratio of polycaprolactone and trifluoroethanol is 10%; (2) preparation of astragaloside solution: astragaloside IV is completely dissolved in dimethyl sulfoxide to make astragaloside A glycoside solution, wherein the mass concentration of the astragaloside IV solution is 0.2g / ml; (3) Preparation of the astragaloside IV polycaprolactone solution: add the astragaloside IV solution to the polycaprolactone solution, and heat the astragaloside IV solution at 37°C Keep stirring until the solution is uniform, wherein the weight-to-volume ratio of astragaloside IV and polyc...
Embodiment 2
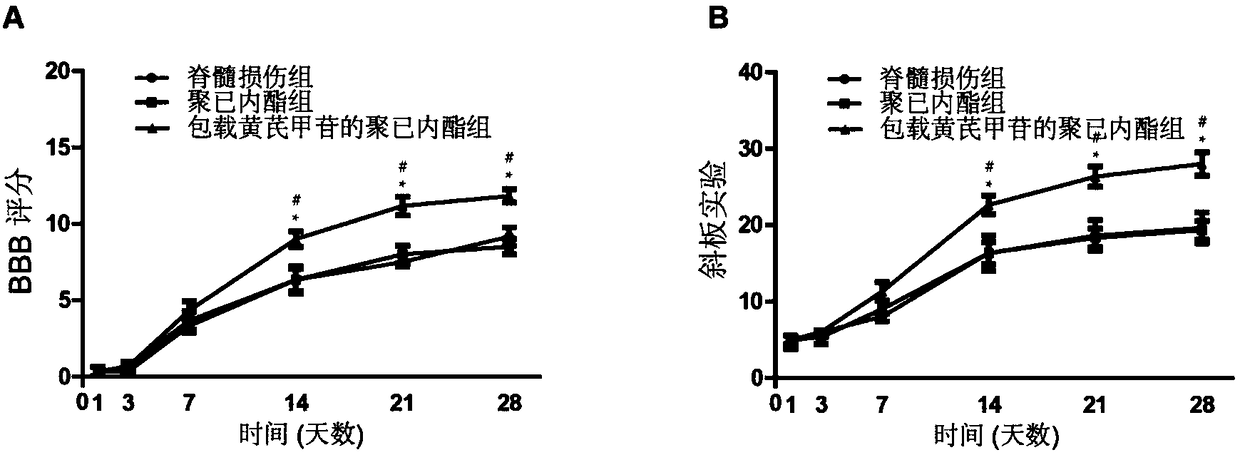
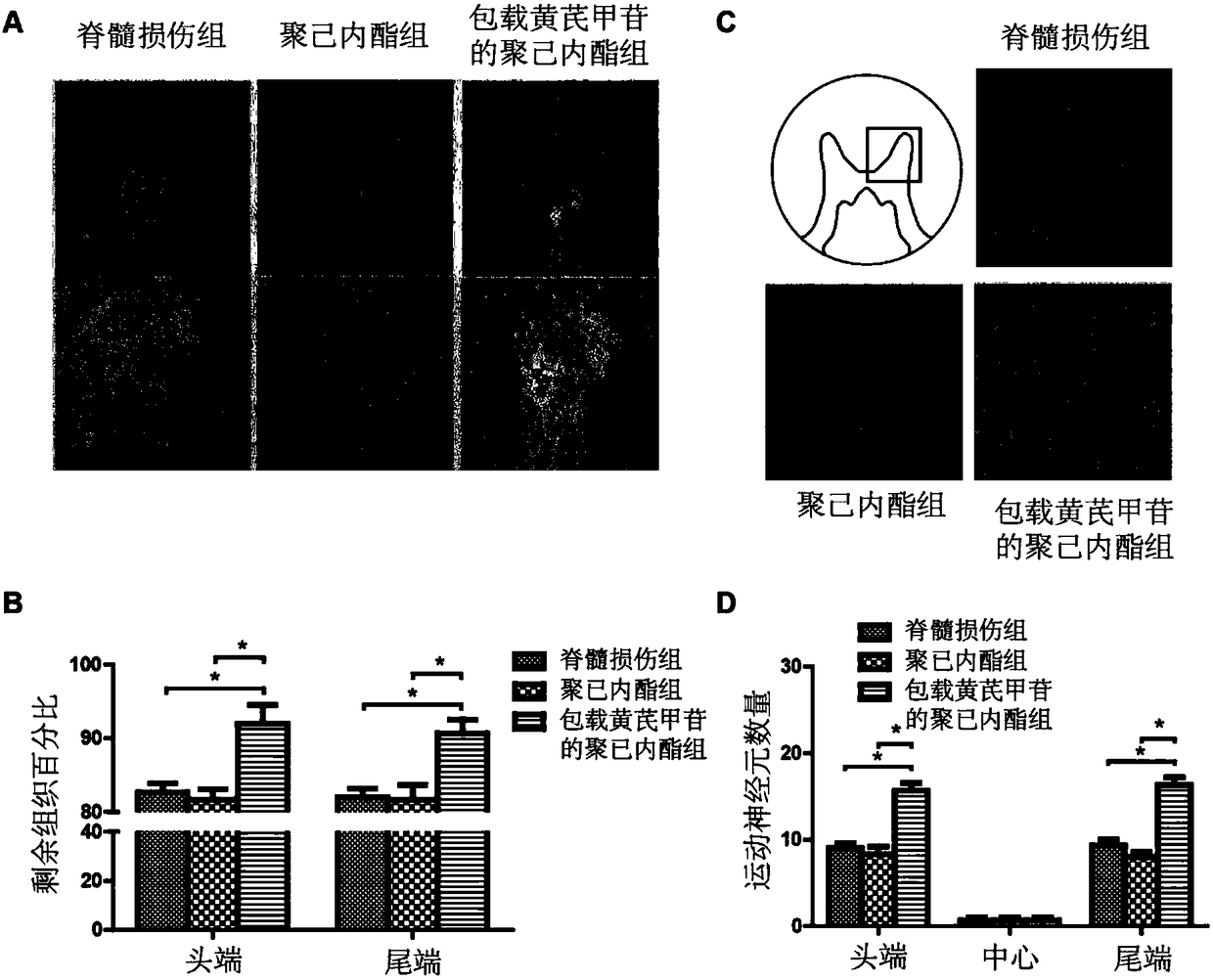
[0062] Application of the polycaprolactone film loaded with astragaloside IV prepared by the preparation method in Example 1
[0063] 1. Animal model establishment:
[0064] ① Anesthesia: Calculated at 65 mg / kg, intraperitoneally anesthetized with 1% sodium pentobarbital.
[0065] ②Prepare the rat spinal cord injury model skin in a sterile environment, disinfect with 2% povidone iodine, make an incision about 0.5 cm along the back, incise layer by layer to expose the thoracic 8-thoracic 11 vertebrae, and use microsurgical instruments The lamina of the 9th thoracic vertebra was removed to expose the spinal cord, and the exposed spinal cord was clamped for 1 minute with a special clamp (clamping force of 30g), sterilized, and sutured after placing a biomaterial membrane. The untreated group was directly sterilized and sutured.
[0066] 2. Grouping of experimental animals:
[0067] (1) Divide 42 SD rats of 220-250 g into 3 groups.
[0068] (2) Control group (spinal cord injur...
Embodiment 3
[0080] Preparation of polycaprolactone film loaded with astragaloside IV
[0081] The preparation of polycaprolactone film loaded with astragaloside includes the following steps: (1) preparation of polycaprolactone solution: polycaprolactone is put into trifluoroethanol solution, stirred overnight at 32 ° C, and prepared into polycaprolactone Caprolactone solution, wherein the weight-to-volume ratio of polycaprolactone and trifluoroethanol is 6%; (2) preparation of astragaloside solution: astragaloside IV is completely dissolved in dimethyl sulfoxide to make astragaloside A glycoside solution, wherein the mass concentration of the astragaloside IV solution is 0.3 g / ml; (3) Preparation of the astragaloside IV polycaprolactone solution: add the astragaloside IV solution to the polycaprolactone solution, and heat the astragaloside IV solution at 32° C. Keep stirring until the solution is uniform, wherein the weight-to-volume ratio of astragaloside IV and polycaprolactone solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com