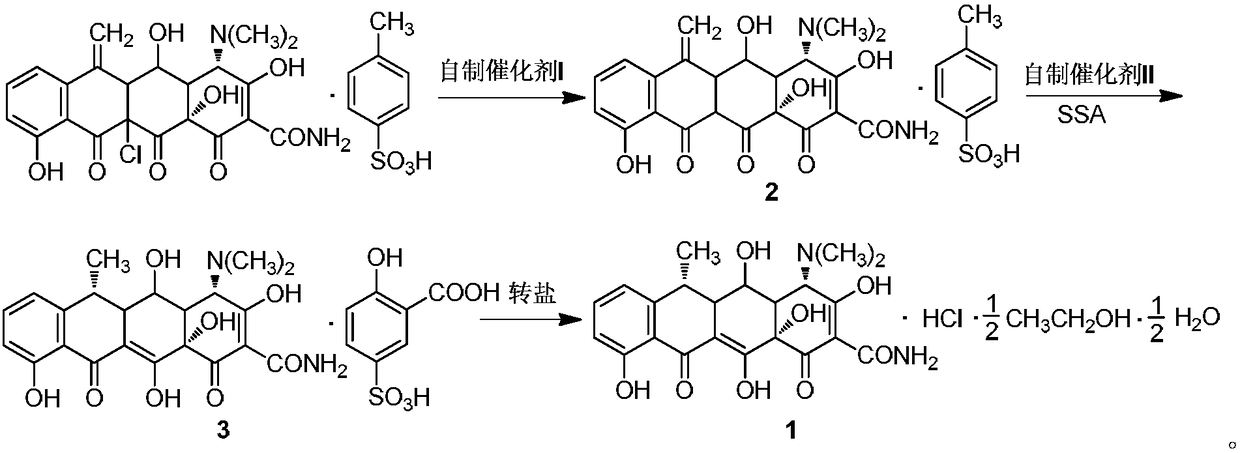
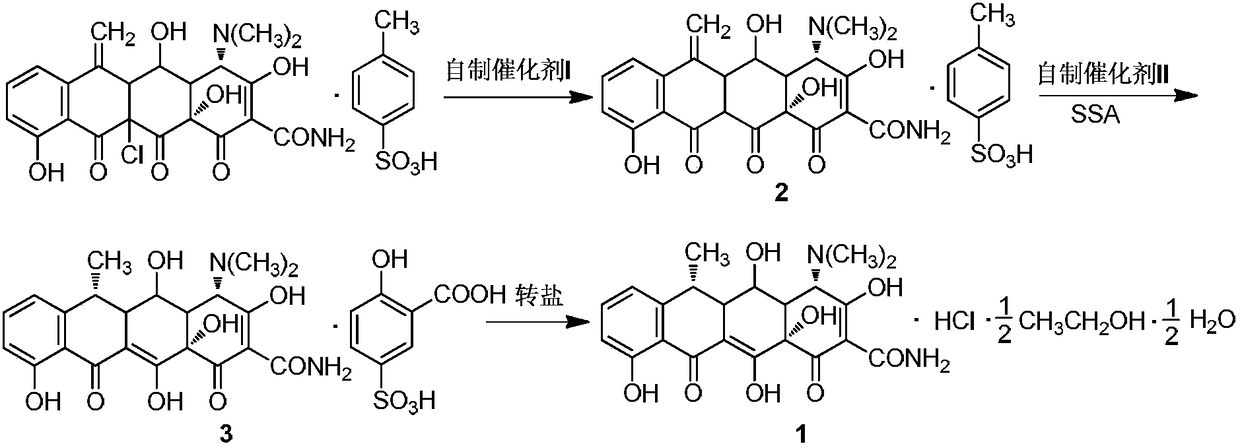
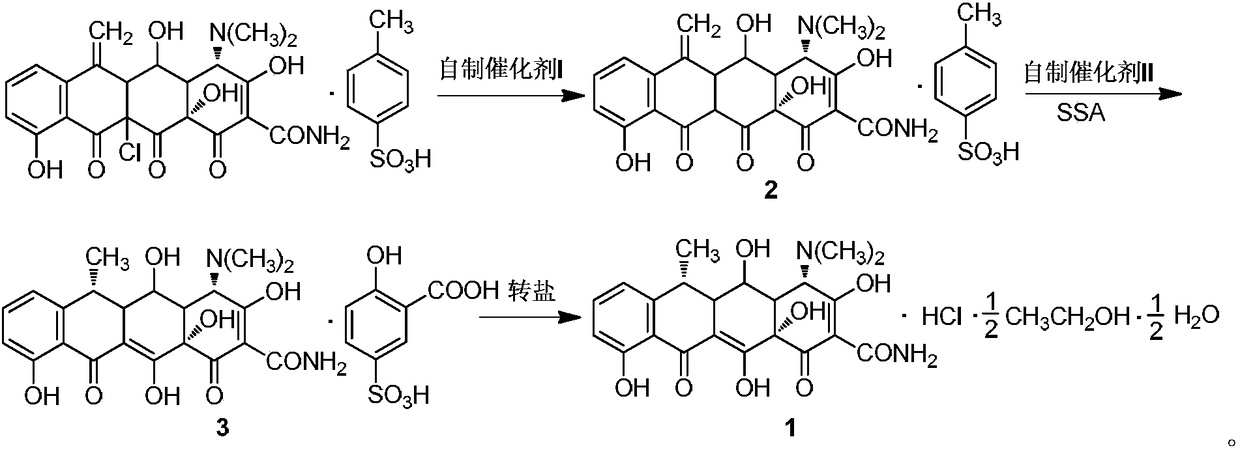
Green high-efficiency synthetic method for doxycycline hydrochloride
A doxycycline hydrochloride, high-efficiency technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of sulfonates, etc., can solve the problems of high cost, low yield, poor stereoselectivity, etc., and achieve green and simple. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Put 1.5g of palladium chloride powder into a beaker containing 250mL of deionized water, fully dissolve it to obtain a palladium chloride solution for use; weigh 4g of sodium hydroxide and put it into a beaker containing 50mL of deionized water, fully Stir to obtain a sodium hydroxide solution for use; add 3.0 g of eicosenoic acid to the beaker of the prepared sodium hydroxide solution, and place the beaker in an ultrasonic cleaner and stir vigorously to fully dissolve the eicosenoic acid until Sodium eicosenoate solution is obtained until there is no massive eicosenoic acid in the solution; heat the prepared 250mL palladium chloride solution to 65°C, and then quickly add the prepared eicosenoic acid solution while stirring the palladium chloride solution Palladium hydroxide solution can be obtained by adding sodium acrylate solution, and the palladium hydroxide solution is continuously stirred for 10 minutes to make the reaction more uniform; 100 mL of hydrogen peroxide...
Embodiment 2
[0024] Put 1.5g of palladium chloride powder into a beaker containing 250mL of deionized water, fully dissolve it to obtain a palladium chloride solution for use; weigh 4g of sodium hydroxide and put it into a beaker containing 50mL of deionized water, fully Stir to obtain a sodium hydroxide solution for use; add 3.0 g of eicosatrienoic acid to the beaker of the prepared sodium hydroxide solution, and place the beaker in an ultrasonic cleaner and stir vigorously to fully dissolve the eicosatrienoic acid , until there is no massive eicosatrienoic acid in the solution to obtain sodium eicosatrienoate solution; heat the prepared 250mL palladium chloride solution to 65°C, and then quickly add the prepared palladium chloride solution while stirring eicosatrienoic acid sodium solution, palladium hydroxide solution can be obtained, keep stirring for 10min to make the reaction more uniform; add 100mL mass concentration of 30% hydrogen peroxide solution to the obtained palladium hydroxi...
Embodiment 3
[0026] Put 1.5g of palladium chloride powder into a beaker containing 250mL of deionized water, fully dissolve it to obtain a palladium chloride solution for use; weigh 4g of sodium hydroxide and put it into a beaker containing 50mL of deionized water, fully Stir to obtain a sodium hydroxide solution for use; add 3.0 g of linoleic acid to the beaker of the prepared sodium hydroxide solution, and place the beaker in an ultrasonic cleaner and stir vigorously to fully dissolve the linoleic acid until there is no lump in the solution The sodium linoleate solution is obtained until the linoleic acid is like linoleic acid; the prepared 250mL palladium chloride solution is heated to 65°C, and then the prepared sodium linoleate solution is quickly added while stirring the palladium chloride solution to obtain the hydroxide Palladium solution, keep stirring for 10min to make the reaction more uniform; add 100mL of hydrogen peroxide solution with a mass concentration of 30% in the obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com