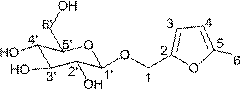
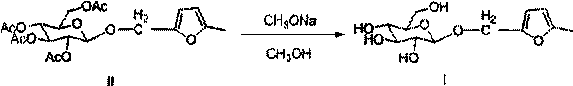Preparation method and application of 5-methyl furfuryl alcohol-beta-D-glucoside
A technology of methyl furfuryl alcohol and glucoside, which is applied in the field of tobacco flavors, can solve the problems of easy volatilization of 5-methyl furfuryl alcohol and easy loss of flavor, and achieve the effects of simple post-processing, reducing miscellaneous gas, and improving volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of 5-methylfurfuryl alcohol-β-D-glucoside of this embodiment is:
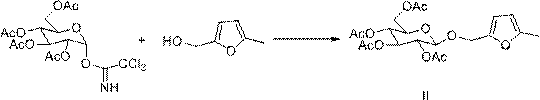
[0038] (1) Synthesis of 5-methylfurfuryl alcohol-β-D-tetraacetyl glucoside (Ⅱ):
[0039] Dissolve 0.56 g (5 mmol) of 5-methylfurfuryl alcohol and 2.98 g (6 mmol) of trichloroacetimide ester sugar in 15 mL of dry dichloromethane, add 1 g of freshly baked 4 Å molecular sieve, and stir at room temperature After 10 min, cool to -20 ℃, add 1.41g (10 mmol) BF 3 •Et 2 O continued to incubate the reaction for 2 h, and TLC detected the progress of the reaction. After the reaction, 30 mL of dichloromethane was added, shaken, and separated. The organic phase was washed 3 times with saturated sodium carbonate solution and 3 times with saturated brine. The organic phase was separated, dried with anhydrous sodium sulfate overnight, filtered, and depressurized. After concentration, it is separated and purified by silica gel column chromatography [ V (Petroleum ether): V (Ethyl acetate)=4:1] to obtain...
Embodiment 2
[0049] The preparation method of 5-methylfurfuryl alcohol-β-D-glucoside of this embodiment is:
[0050] (1) Synthesis of 5-methylfurfuryl alcohol-β-D-tetraacetyl glucoside (Ⅱ):
[0051] Dissolve 0.56 g (5 mmol) of 5-methylfurfuryl alcohol and 4.96 g (10 mmol) of trichloroacetimide ester sugar in 20 mL of dry dichloromethane, add 2 g of freshly baked anhydrous calcium sulfate, at room temperature Stir for 10 min and then cool to -20 ℃, add 2.12 g (15 mmol) BF 3 •Et 2 O continued to incubate the reaction for 1 h, and TLC checked the progress of the reaction. After the reaction, add 30 mL of dichloromethane, shake, and separate. The organic phase was washed 4 times with saturated sodium carbonate solution and 4 times with saturated brine. The organic phase was separated, dried with anhydrous sodium sulfate overnight, filtered, and depressurized. After concentration, it is separated and purified by silica gel column chromatography [ V (Petroleum ether): V (Ethyl acetate)=4:1], 1.70 g ...
Embodiment 3
[0057] The preparation method of 5-methylfurfuryl alcohol-β-D-glucoside of this embodiment is:
[0058] (1) Synthesis of 5-methylfurfuryl alcohol-β-D-tetraacetyl glucoside (Ⅱ):
[0059] Dissolve 0.56 g (5 mmol) of 5-methylfurfuryl alcohol and 3.72 g (10 mmol) of trichloroacetimide ester sugar in 20 mL of dry dichloromethane, add 2 g of freshly baked anhydrous calcium sulfate, at room temperature Stir for 10 min and then cool to -20 ℃, add 1.41 g (15 mmol) BF 3 •Et 2 O continued to incubate the reaction for 2 h, and TLC detected the progress of the reaction. After the reaction, add 30 mL of dichloromethane, shake, and separate. The organic phase was washed 5 times with saturated sodium carbonate solution and 5 times with saturated brine. The organic phase was separated, dried with anhydrous sodium sulfate overnight, filtered, and depressurized. After concentration, it is separated and purified by silica gel column chromatography [ V (Petroleum ether): V (Ethyl acetate)=3:1], 1.75g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com