Solid-phase-core coating recombinant lipoprotein, preparation and applications thereof
A technology of solid-phase core and apolipoprotein, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, drug combinations, etc., and can solve the problem of limited loading method, physical and chemical properties of drug molecules, low loading capacity, etc. problems, to achieve the effect of improving load capacity and stability, and not easy to gather
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. Preparation and Characterization of Recombinant Lipoprotein Containing Calcium Phosphate Solid Phase Core
[0047] (1) Preparation
[0048] The calcium phosphate solid-phase inner core is prepared by a reverse microemulsion method, and the reverse microemulsion is prepared by dispersing an aqueous solution into a cyclohexane oil phase solution containing nonylphenol polyoxyethylene ether. First, 300 μL of 2.5 M CaCl 2 The solution is dispersed in 20mL oil phase to form a uniformly dispersed water-in-oil reverse microemulsion, and this step is the preparation of the calcium phase. The phosphorus phase was prepared by dissolving 300 μL of 12.5 mM Na 2 HPO 4 The solution was dispersed in another 20 mL oil phase, and after stirring for 10 min, 100 μL of 20 mg / mL 1,2-dioleoyl phosphatidic acid (DOPA) solution was added to the phosphorus phase. After the two phases were evenly dispersed, the two phases were mixed and stirred for 45 min. After the microemulsion...
Embodiment 2
[0053] Example 2. Preparation of recombinant lipoprotein containing calcium carbonate solid phase core
[0054] The calcium carbonate solid-phase core is prepared by a reverse microemulsion method, and the reverse microemulsion is prepared by dispersing an aqueous solution into a cyclohexane oil phase solution containing nonylphenol polyoxyethylene ether. First add a small amount of CaCl 2 The solution is dispersed in the oil phase to form a uniformly dispersed water-in-oil inverse microemulsion, and this step is the preparation of the calcium phase. The carbon phase is prepared by Na 2 CO 3 The solution was dispersed in another oil phase, and after stirring for 10 min, 100 μL of 20 mg / mL 1,2-dioleoylphosphatidic acid (DOPA) solution was added to the carbon phase. After the two phases were evenly dispersed, the two phases were mixed and stirred for 45 min. After the microemulsions react after exchange, calcium carbonate precipitation will be produced. At this time, 40 mL ...
Embodiment 3
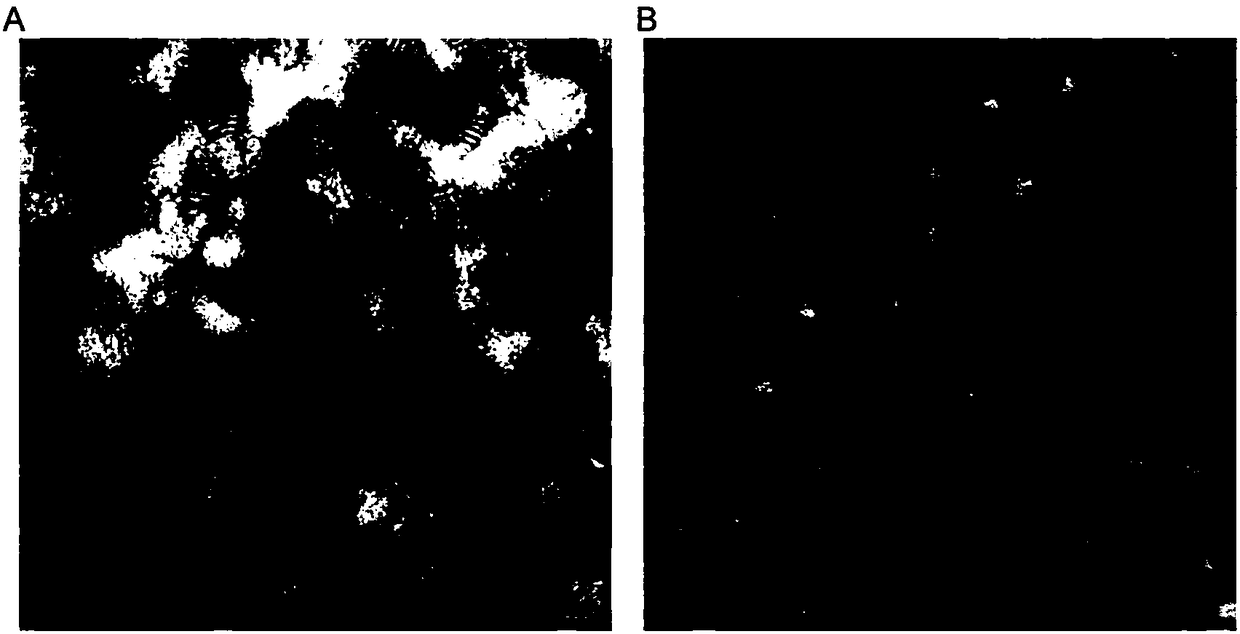
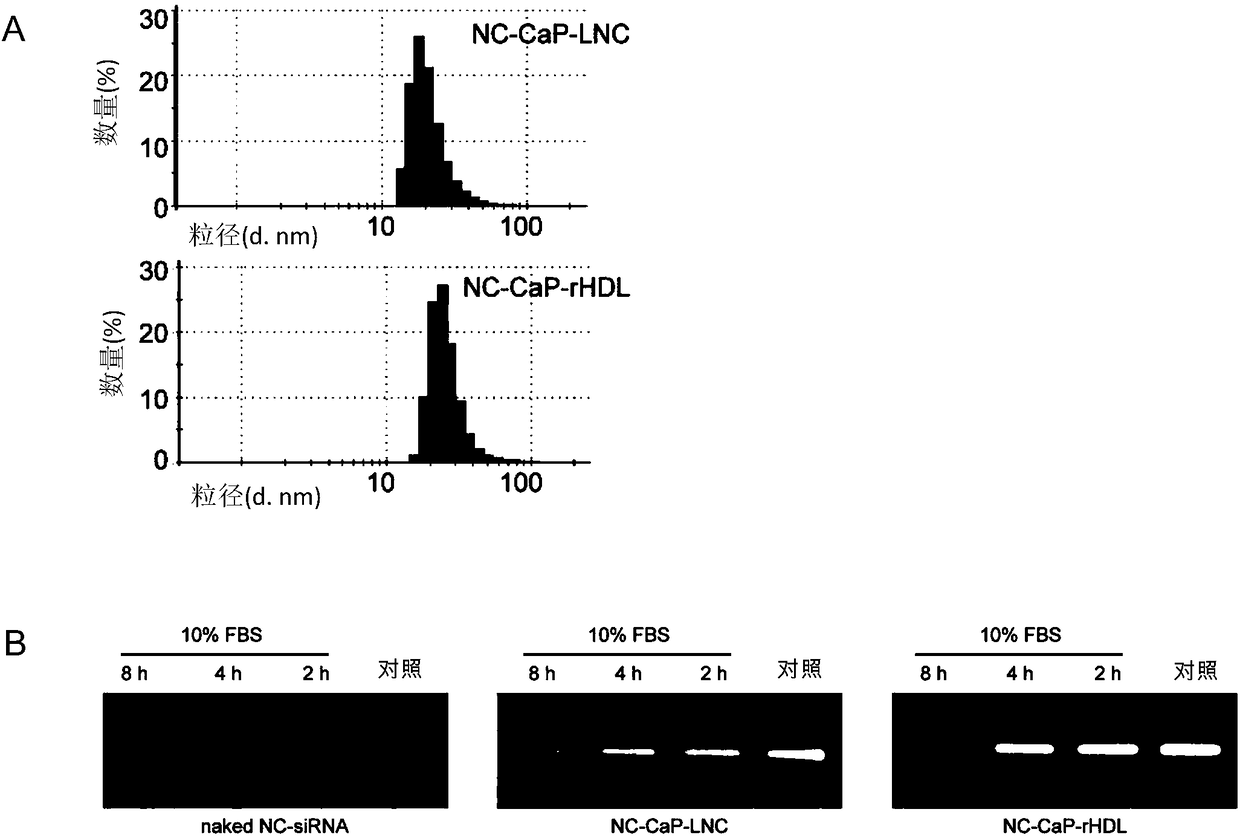
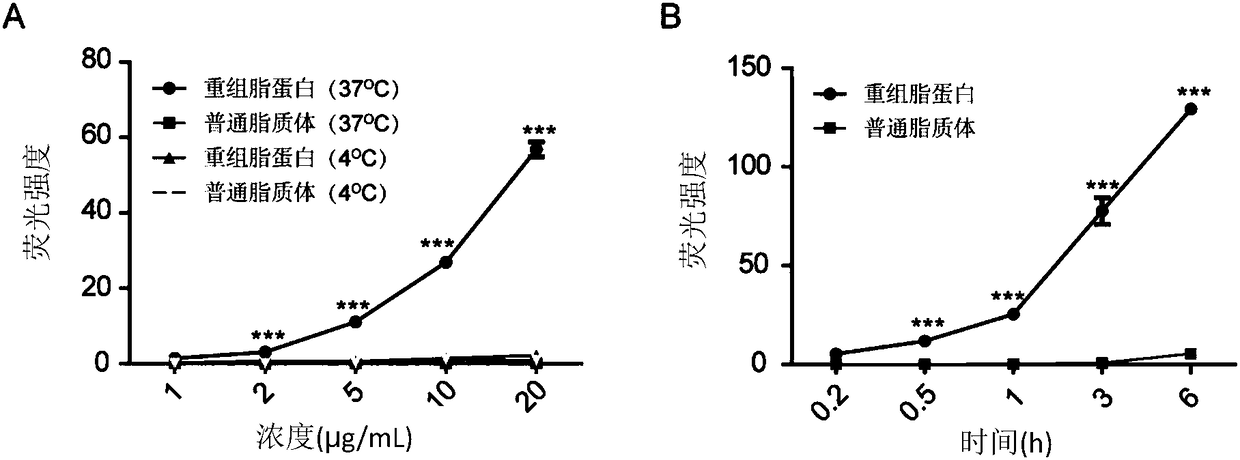
[0056] Example 3. Incubate the recombinant lipoprotein of the calcium phosphate solid-phase inner core of NC siRNA or ordinary liposomes, and co-incubate with 10% FBS, evaluate the stability of the recombinant lipoprotein of the NC siRNA calcium phosphate solid-phase inner core
[0057] (1) Preparation
[0058] The calcium phosphate solid-phase inner core is prepared by the inverse microemulsion method as in Example 1. First, 300 μL of CaCl with a concentration of 2.5M 2 The solution was mixed with 80 μL of 2 mg / mL NC siRNA solution and then dispersed in 20 mL of oil phase to form a uniformly dispersed water-in-oil inverse microemulsion. This step was the preparation of the calcium phase. The subsequent preparation method was the same as that in Example 1. The calcium phosphate solid-phase core loaded with NC siRNA and DOPA was prepared, dispersed in 1 mL of chloroform and stored in a glass bottle for subsequent experiments.
[0059] Prepare ordinary liposomes containing calc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com