Arginine glutamate injection pharmaceutical composition and preparation method thereof
A technology of arginine glutamic acid and composition, applied in the field of medicine, can solve the problems of high content of main impurities, increased risk of adverse reactions of patients, difficult control of preparation quality, etc., and achieves the effect of easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
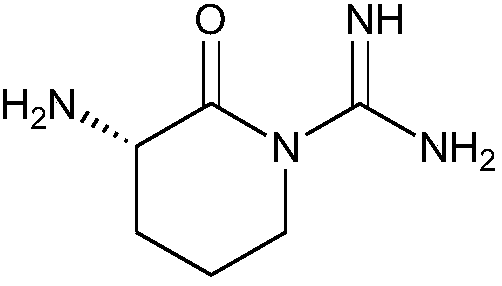
[0032] The preparation of preparation example 1 main impurity acetate:
[0033] Take an appropriate amount of arginine acetate, add concentrated ammonia water to dissolve completely. Reflux at 100°C for 25 hours, add an appropriate amount of concentrated ammonia water every 4 hours, follow and monitor the reaction by HPLC, until the peak area of arginine in the HPLC is normalized to account for about 0.5%. At 100°C, add 0.1% activated carbon and filter. The filtrate was rotary evaporated to dryness, added methanol, cooled to 0°C, filtered to obtain the main impurity acetate, off-white powder, and the purity was greater than 99.0% by HPLC analysis. The chemical characteristics of the main impurity acetate are as follows:
[0034] Molecular formula: C 8 h 16 N 4 o 3 ; MS m / z (int): 157 [M+H-CH 3 COOH]; 1 H-NMR (DMSO-d6, 800MHz): δ8.23 (1H, s, NH), 5.50 (3H, brs, NH3), 6.04 (2H, brs, NH 2 ), 3.11 (1H,m,CH-NH 3 ), 3.10 (2H, m, CH 2 -N), 1.4-2.1(4H,m,CH 2 -CH 2 ), 1....
preparation example 2
[0035] The preparation of preparation example 2 reference substance solution
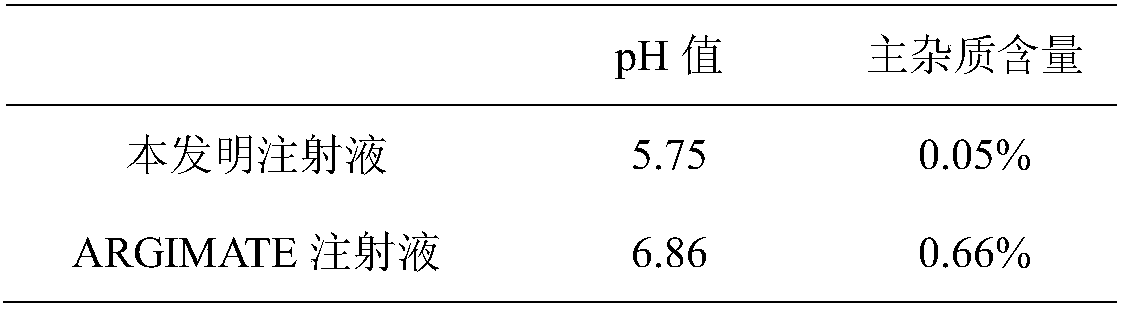
[0036] Take an appropriate amount of the pharmaceutical composition of arginine glutamic acid injection, dilute it 10 times with water, and dilute it with mobile phase to a solution containing about 5 mg per 1 mL or directly use mobile phase to a solution containing about 5 mg per 1 mL. The reference substance solution was prepared by diluting the above solution 100 times.
preparation example 3
[0037] The preparation of preparation example 3 main impurity reference substance solution
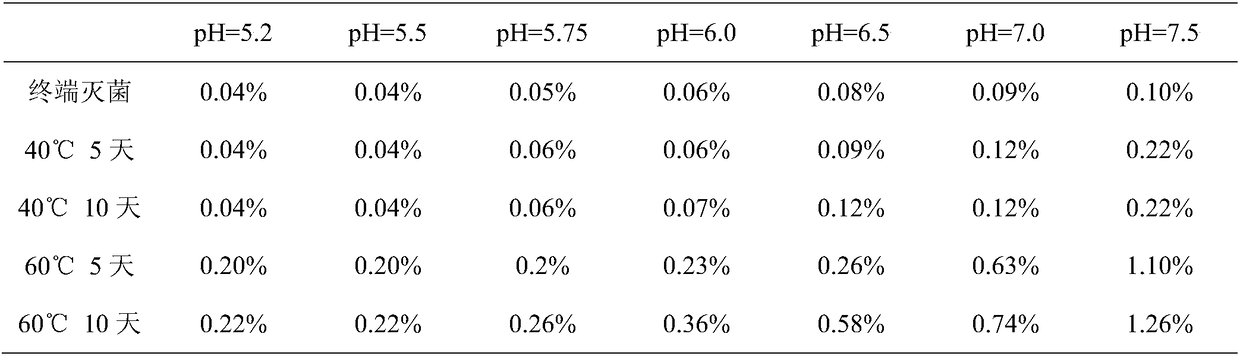
[0038] Take an appropriate amount of the main impurity reference substance, add mobile phase and dilute it into a solution containing about 2 mg per 1 mL (calculated as lactam), as the main impurity control stock solution, and prepare the impurity reference solution according to the impurity content in the sample during use (the main impurity content is between The limit of quantitation is within the range of 0.005%, and the stock solution is diluted into a control solution containing 0.1 μg of the main impurity per 1 mL;
[0039] In the range of 0.0025% to 0.05%, the stock solution is diluted to a main impurity control solution containing 1 μg per 1 mL; above 0.05%, the stock solution is diluted to a main impurity control solution containing 20 μg per 1 mL).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com