System and method for producing fuel chemicals by conducting solar high-temperature thermal-coupling on methane
A chemical, solar technology, applied in the field of zero carbon emission system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
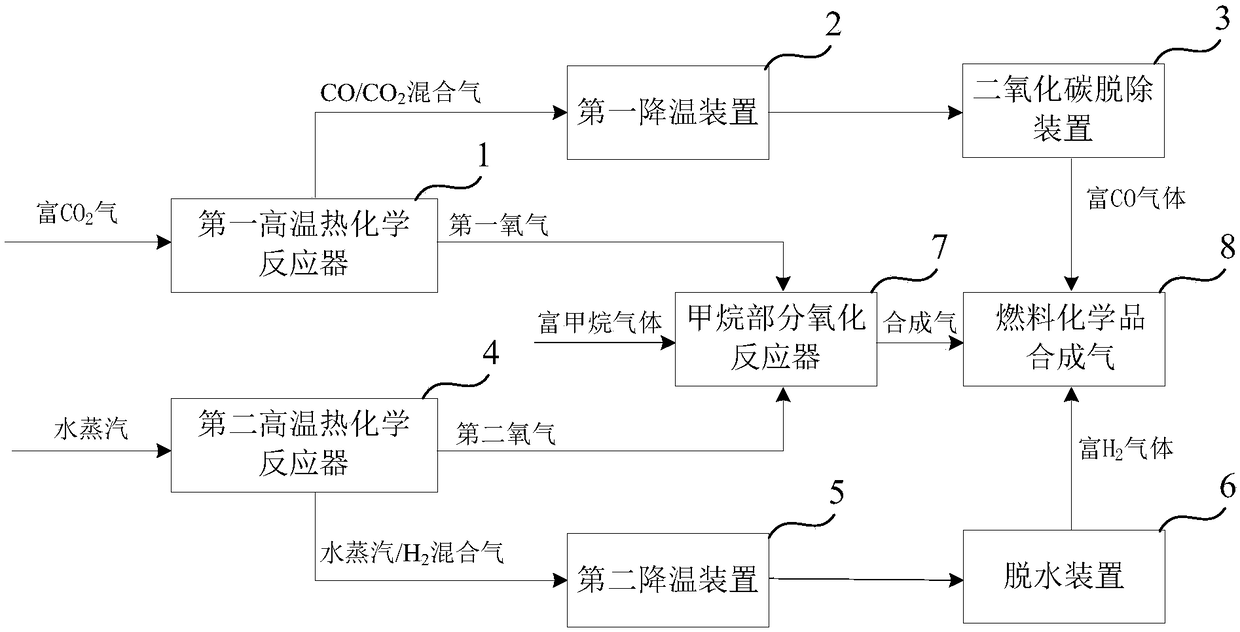
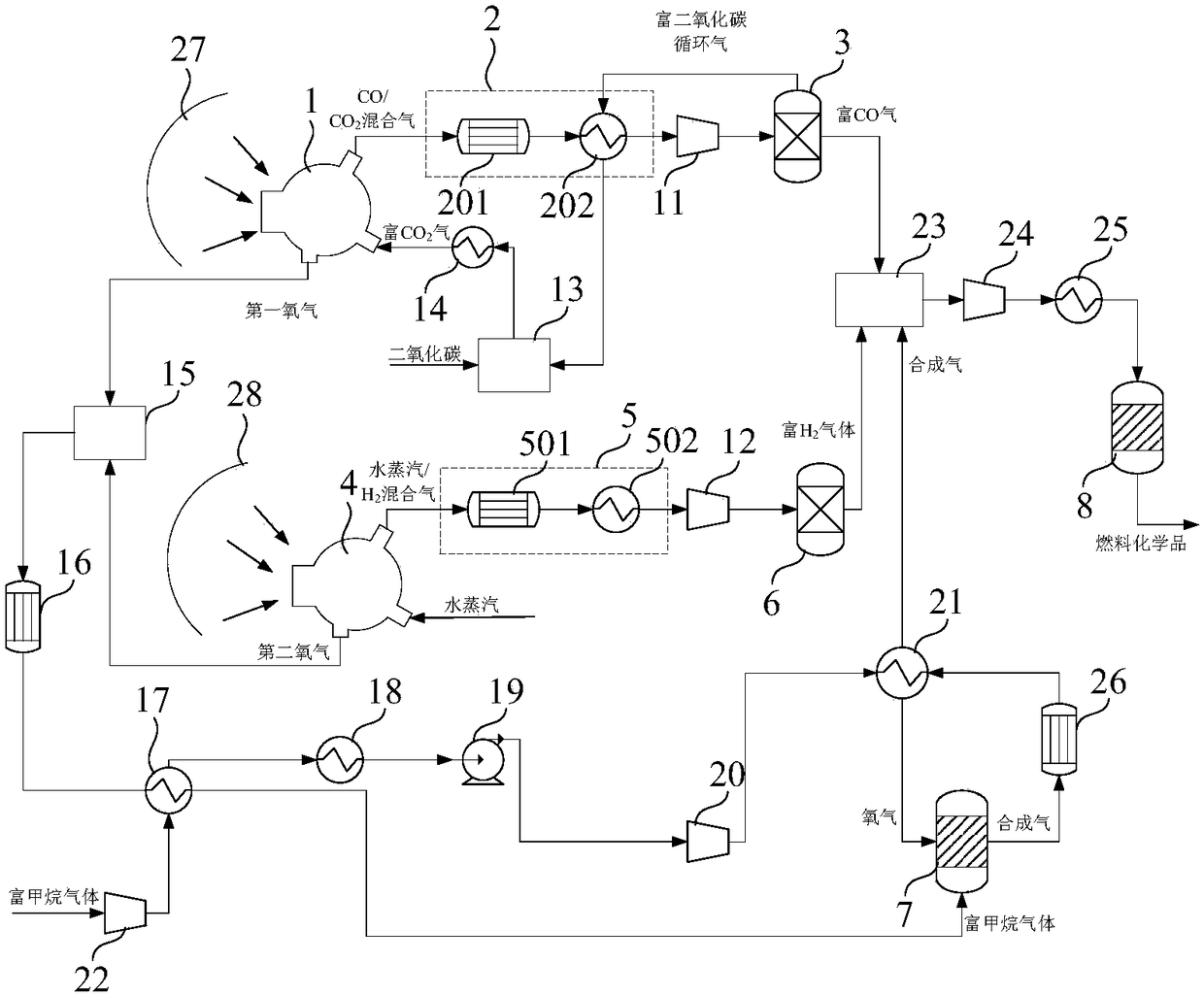
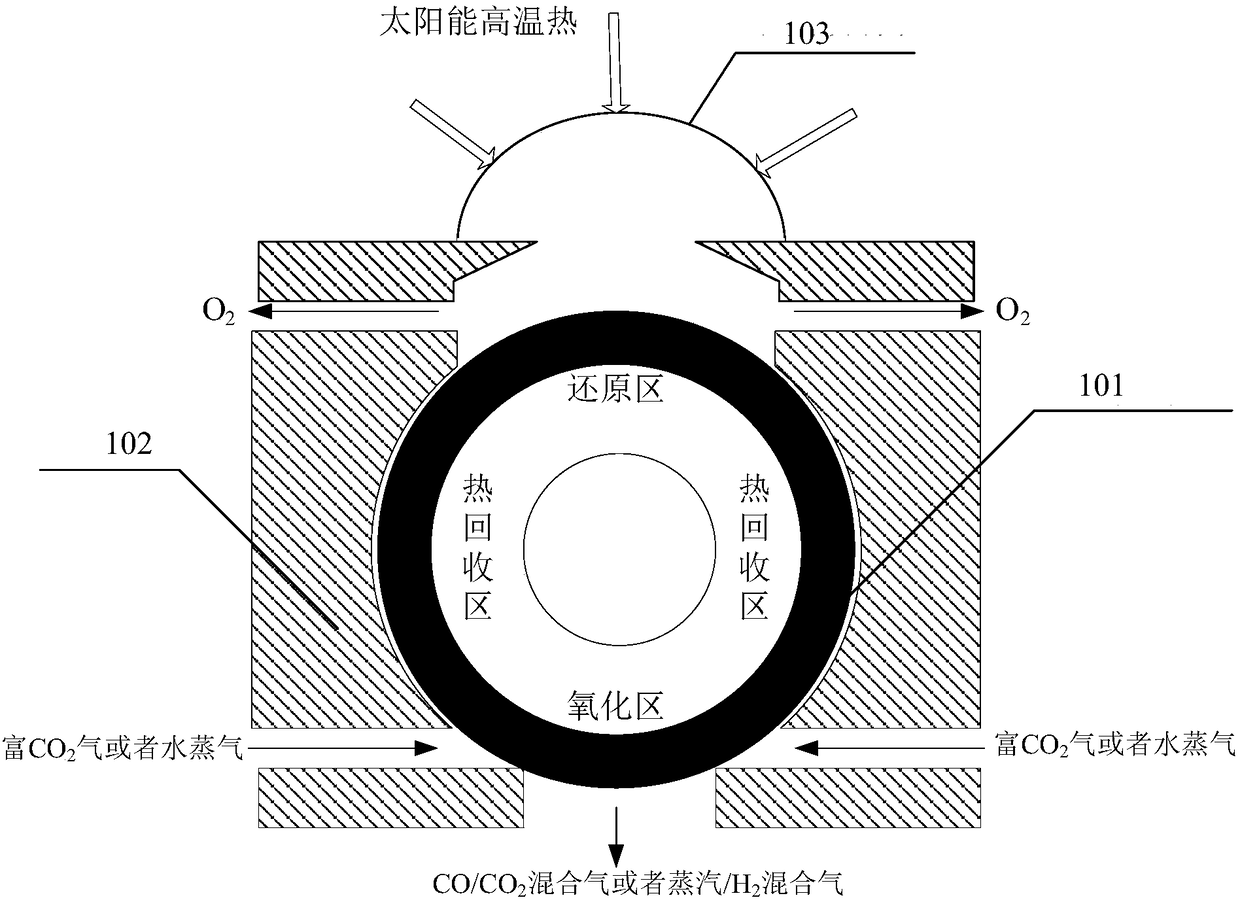
[0147] The sunlight collected from the heliostat field passes through the first CPC secondary concentrator 27, and the heat is 147381.1kW, and then enters the first high-temperature thermochemical reactor 1 through the quartz glass cover covered on the first high-temperature thermochemical reactor 1 The operating pressure in the reaction chamber is 0.2atm, the temperature in the reduction reaction zone is 1350-1400°C, and the temperature in the oxidation reaction zone is 1050-1100°C. Fresh CO required for the first high temperature thermochemical reactor 1 2 The amount is 96.4kmol / hr, the CO / CO at the outlet of the oxidation reaction zone 2 After the mixed gas is separated, the yield of CO-rich gas is 96.4kmol / hr, the molar content of CO is 84%, and the O at the outlet of the reduction zone 2 The (first oxygen) production was 41.3 kmol / hr.
[0148] The sunlight collected from the heliostat field passes through the second CPC secondary concentrator 28, and the heat is 147381....
Embodiment example 2
[0150] The sunlight collected from the heliostat field passes through the first CPC secondary concentrator 27, and the heat is 369.5MW, and enters the first high-temperature thermochemical reactor 1 through the quartz glass cover covered on the first high-temperature thermochemical reactor 1 In the reaction chamber, the operating pressure in the reaction chamber is 0.2atm, the temperature in the reduction reaction zone is 1350-1400°C, and the temperature in the oxidation reaction zone is 1050-1100°C. Fresh CO required for the first high temperature thermochemical reactor 1 2 The amount is 340.8kmol / hr, the CO / CO at the outlet of the oxidation reaction zone 2 After the mixed gas is separated, the yield of CO-rich gas is 340.8kmol / hr, the CO content is 87%, and the O at the outlet of the reduction zone 2 The (first oxygen) production was 148.2 kmol / hr.
[0151] The heat from the sunlight collected by the heliostat field is 369.5MW after passing through the second CPC secondary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com