Thioureido modified imidazoline derivative corrosion inhibitor and preparation method and application of thioureido modified imidazoline derivative corrosion inhibitor
A technology based on imidazoline and thiourea, which is applied in the field of thiourea-modified imidazoline derivative corrosion inhibitors and its preparation, can solve the problems of poor application of corrosion, poor film-forming properties, and few electron-donating groups. Good corrosion inhibition effect, good fluidity and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
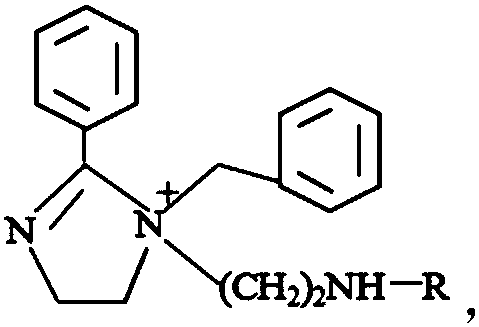
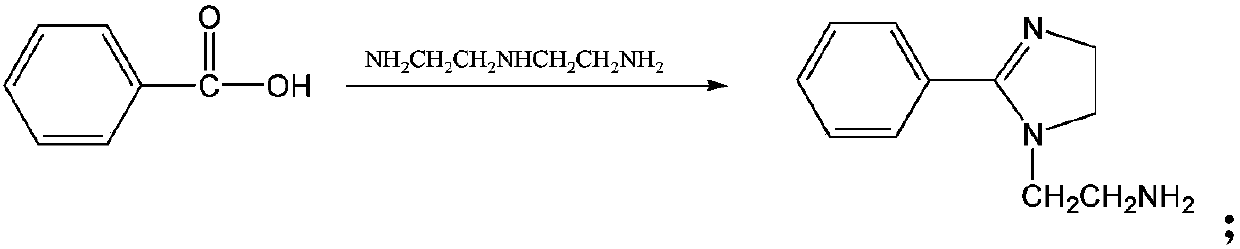
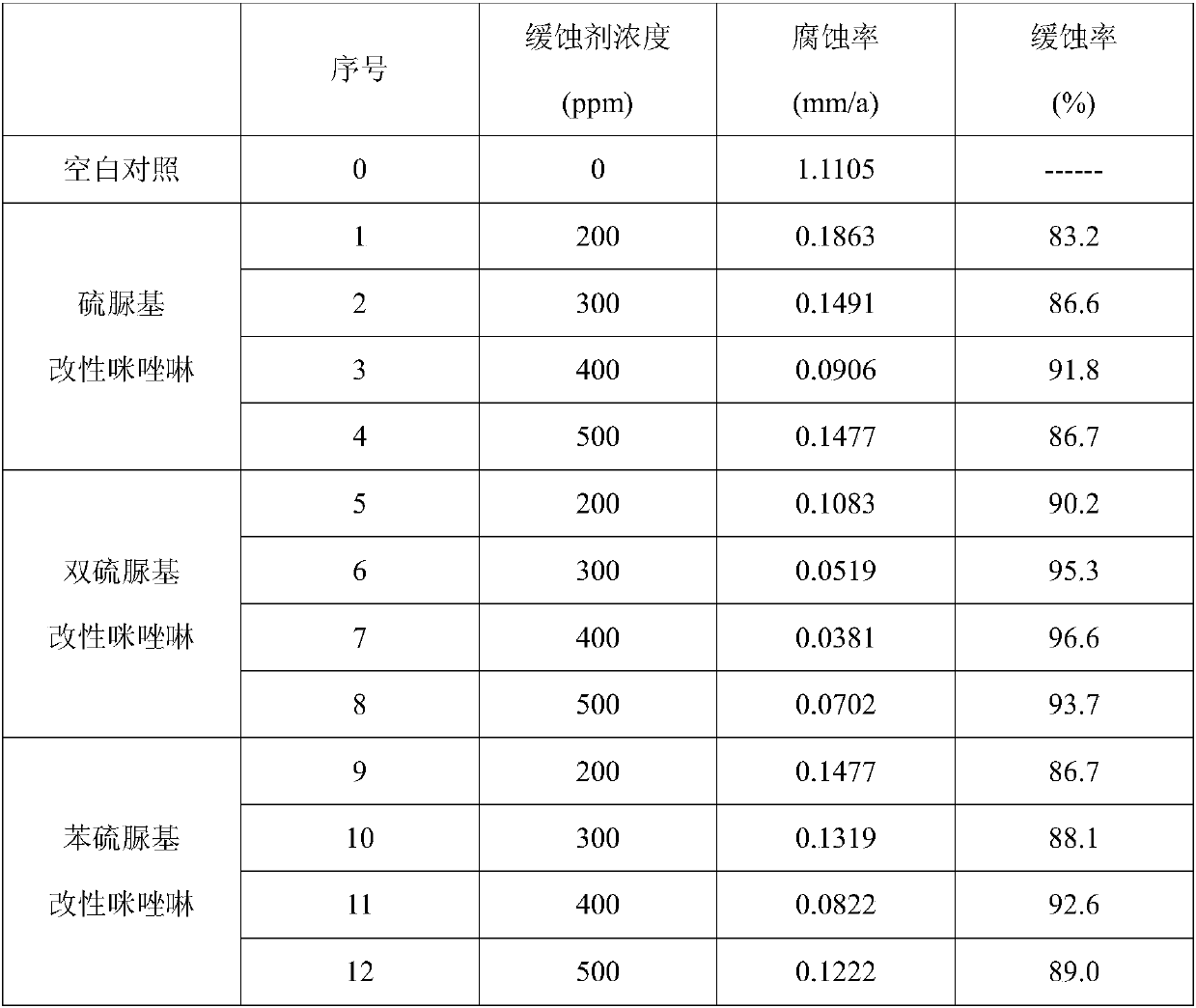
[0035] This embodiment provides a preparation method of a thiourea-based modified imidazoline derivative corrosion inhibitor. The thiourea group-modified imidazoline is obtained by reacting benzoic acid and diethylenetriamine to obtain an imidazoline intermediate, then reacting with benzyl chloride to obtain an imidazoline quaternary ammonium salt, and finally reacting with thiourea.
[0036] The present embodiment provides the specific synthesis steps of the above-mentioned modified imidazoline, and the specific steps are as follows:
[0037] Add benzoic acid and diethylenetriamine into the container, and in the presence of water-carrying agent xylene, benzoic acid (1 mol) and diethylene triamine (1.3 mol) are heated gradually, and the reaction is carried out at 100° C. for 2 hours; The temperature was then raised to 210°C for cyclization reaction, 4h, to obtain the imidazoline intermediate; cooled to 80°C, benzyl chloride (1.2mol) was slowly added dropwise for quaternization...
Embodiment 2
[0040] This embodiment provides a preparation method of a bisthiourea-based modified imidazoline derivative corrosion inhibitor. The dithiourea group-modified imidazoline is obtained by reacting benzoic acid and diethylenetriamine to obtain an imidazoline intermediate, then reacting with benzyl chloride to obtain an imidazoline quaternary ammonium salt, and finally reacting with thiourea.
[0041] The present embodiment provides the specific synthesis steps of the above-mentioned modified imidazoline, and the specific steps are as follows:
[0042]Add benzoic acid and diethylenetriamine into the container, and in the presence of water-carrying agent xylene, benzoic acid (1 mol) and diethylene triamine (1.3 mol) are heated gradually, and the reaction is carried out at 100° C. for 2 hours; The temperature was then raised to 210°C for cyclization reaction, 4h, to obtain the imidazoline intermediate; cooled to 80°C, benzyl chloride (1.2mol) was slowly added dropwise for quaterniza...
Embodiment 3
[0045] This embodiment provides a preparation method of a phenylthiourea-based modified imidazoline derivative corrosion inhibitor. The phenylthiourea group-modified imidazoline is obtained by reacting benzoic acid and diethylenetriamine to obtain an imidazoline intermediate, then reacting with benzyl chloride to obtain an imidazoline quaternary ammonium salt, and finally reacting with phenylthiourea.
[0046] The present embodiment provides the specific synthesis steps of the above-mentioned phenylthiourea group-modified imidazoline, and the specific steps are as follows:
[0047] Add benzoic acid and diethylenetriamine into the container, and in the presence of water-carrying agent xylene, benzoic acid (1 mol) and diethylene triamine (1.3 mol) are heated gradually, and the reaction is carried out at 100° C. for 2 hours; The temperature was then raised to 210°C for cyclization reaction, 4h, to obtain the imidazoline intermediate; cooled to 80°C, benzyl chloride (1.2mol) was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com