Method for producing a lithium battery material, materials and lithium battery
A battery and electrode technology, applied in the field of devices including batteries and lithium batteries according to the present invention, can solve problems such as fluorine and carbon loss, and achieve the effect of improving electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0065] Example 1: Example of implementing the method of the present invention
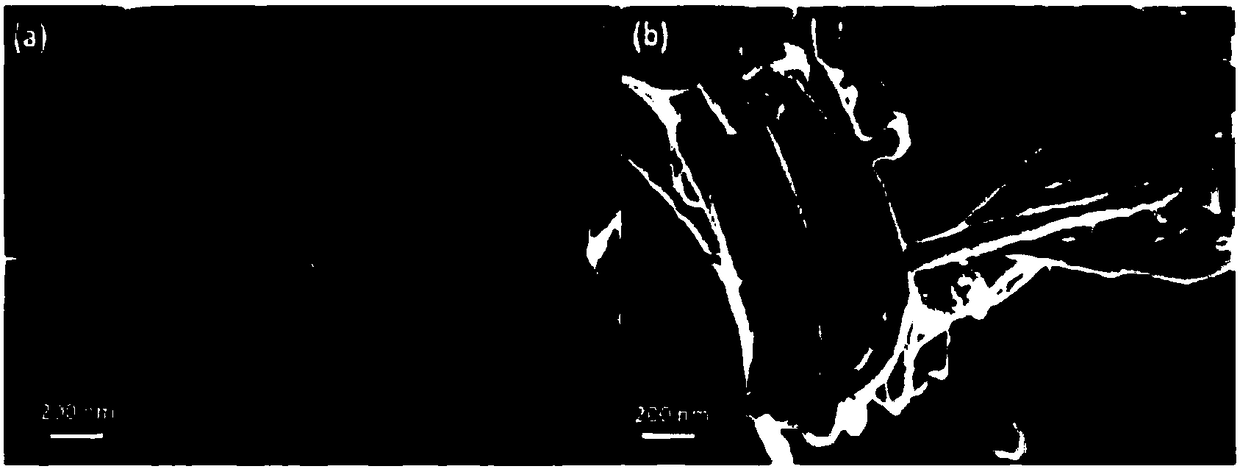
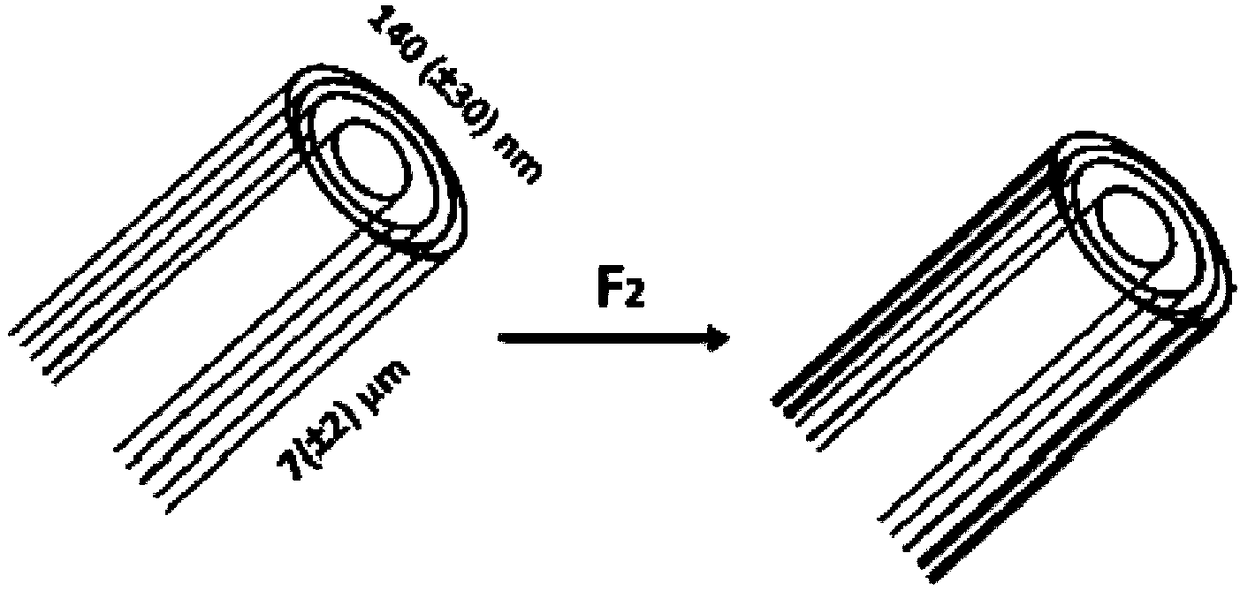
[0066]In said first example, 200 mg of nanofibers (from the company MER, under the commercial reference MRCSD) were placed in a nickel basket positioned in the center of a one-liter passivated nickel reactor to pass through dynamic fluorination (F 2 gas flow in an open reactor) to be fluorinated.
[0067] Dynamic fluorination of pure gaseous molecular fluorine F at a temperature Tf of 420°C 2 It was carried out under flow at a flow rate of 25-30 mL / min for 180 minutes.
[0068] The obtained material had an atomic specific velocity F / C of 0.31 as determined by NMR.
[0069] The material has been milled by a Retsch PM100 (trademark) planetary ball mill in a 50 mL stainless steel vessel with four 10 mm diameter stainless steel balls under an argon atmosphere at a grinding speed of 350 rpm for 9 minutes and 1 minute stop. Hour.
[0070] These experiments were repeated with different CFx materials, ...
example 2
[0077] Example 2: Examples of configurations of electrochemical cells that can be used to practice materials of the invention
[0078] The material obtained according to Example 1 was suspended in a stirrer with a glass vessel by mixing 10% by weight of polyvinylidene fluoride (PVDF) in a propylene carbonate liquid medium.
[0079] The suspension is then deposited on approximately 1 cm of a stainless steel electrode plate heated to 80°C 2 Apparently, the plate was then evacuated under low vacuum at 120° C. for one hour. 50 mg of cathode material CFx / PVDF 90 / 10 were obtained.
[0080] The mass of the plate is then estimated to be between 2 and 5 mg. The plates were returned to the glove box and incorporated into a laboratory lithium battery consisting of lithium metal and a Kargold (registered trademark) microporous separator, filled with LiPF 6 Wetting with PC / EC / 3 DMC 1M electrolyte, as attached Figure 4 shown in . The laboratory cell was a 12 mm internal diameter 304L ...
example 3
[0091] Example 3: Examples of materials obtained according to the method of the invention and comparison with corresponding materials of the prior art
[0092] The examples present different experiments carried out on different materials obtained according to the method of the invention. In particular, the materials tested in this example were obtained using the method of the invention as described in example 1, and the electrochemical performance was measured using the apparatus as described in example 2.
[0093] The potential change is then measured as a function of time. The duration is multiplied by the current density so that the capacity is determined. In 3 electrochemical tests carried out on the material, capacities of 970mAh / g, 1684mAh / g and 2339mAh / g were obtained. The "theoretical" capacity of said material, corresponding to the capacity measured on the fluorinated carbon nanofibrous material used to carry out the method of the invention, ie before grinding, was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com