Method for preparing catechol compounds by biotransformation
A catechol and biotransformation technology, which is applied in the biological high field, can solve the problems of poor applicability of the biotransformation method, and achieve the effect of wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The construction of embodiment 1 aniline oxidase expression bacterial strain
[0017] The gene cluster atdAlA2A3A4A5 encoding ATD was synthesized with reference to the sequence in GenBank (accession number AB008831.1), and then the synthetic DNA fragment was connected to the NdeI and EcoRI sites of the expression vector pET28a (purchased from Treasure Bioengineering Co., Ltd.) to obtain the expression vector pET-atd. Similarly, the gene cluster adoQTA1A2B encoding ADO was synthesized with reference to the sequence in GenBank (accession number CPO10956.1), and then the synthetic DNA fragment was connected to the NdeI and XhoI sites of the expression vector pET28a to obtain the expression vector pET-ado. Transform pET-atd and pET-ado into Escherichia coli BL21(DE3) (purchased from Treasure Bioengineering Co., Ltd.), respectively, to obtain expression strains BL21-ATD and BL21-ADO. The transcription of atdAlA2A3A4A5 and adoQTA1A2B is controlled by the T7 promoter.
Embodiment 2
[0018] The cultivation of embodiment 2 bacterial cells
[0019] The fermentation medium of bacterial strain BL21-ATD and BL21-ADO is glucose 1%, (NH 4 ) 2 SO 4 0.4%, K 2 HPO 4 0.22%, KH 2 PO 4 0.3%, MgSO 4 0.01%, NaCl 0.01%, fish meal 0.2%, yeast extract 0.01%, 1 times trace element mother solution, pH 7.0. The culture temperature was 37°C. In the middle and late stages of logarithmic growth, 0.1% lactose was added to induce the synthesis of ATD or ADO, and the induction time was 6 hours. After induction, the bacterial cells were collected by centrifugation and washed 3 times with pure water.
Embodiment 3
[0020] Embodiment 3 biotransformation method produces 4,5-dichlorocatechol
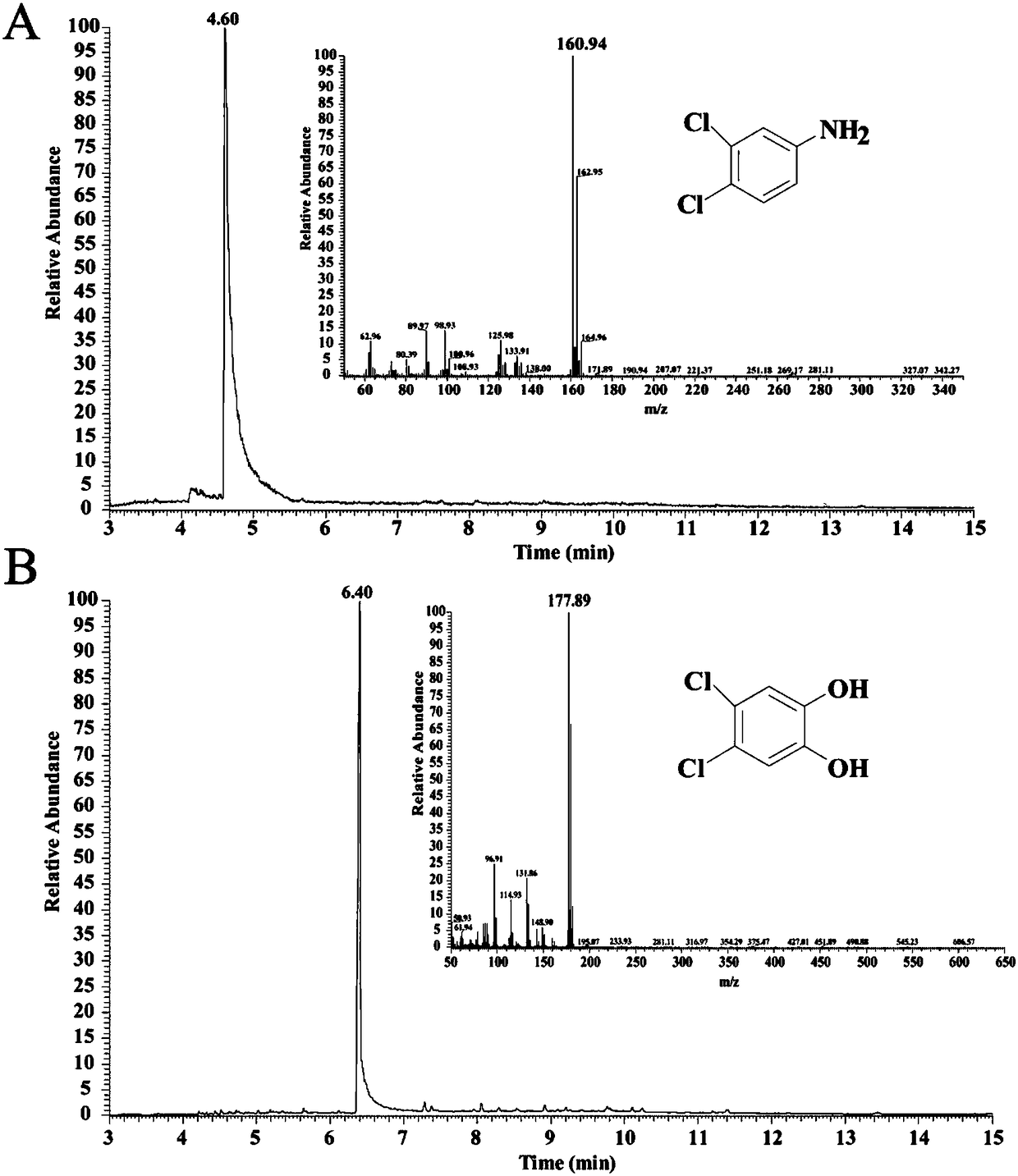
[0021] Using pure water as a solvent, the amount of Escherichia coli cells that synthesized aniline oxidase ADO was 10 9 CFU / ml, the amount of 3,4-dichloroaniline added is 0.4g / L, the reaction temperature is 30°C, the rotation speed is 150 rpm, the reaction time is 12 hours, the bacteria are removed by centrifugation, and the supernatant is concentrated by distillation The product 4,5-dichlorocatechol was obtained with a conversion rate of 95%. As shown in Figure 2, only raw material 3,4-dichloroaniline ( figure 1 A), after the reaction, only product 4,5-dichlorocatechol ( figure 1 B). The bacterial cells removed by centrifugation were harmlessly treated by steam sterilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com