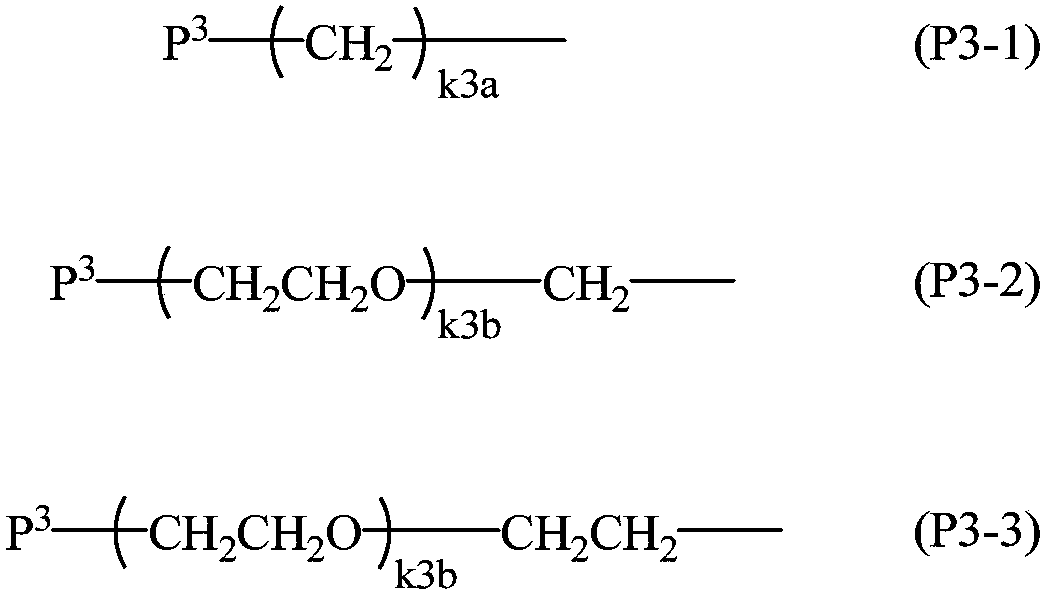
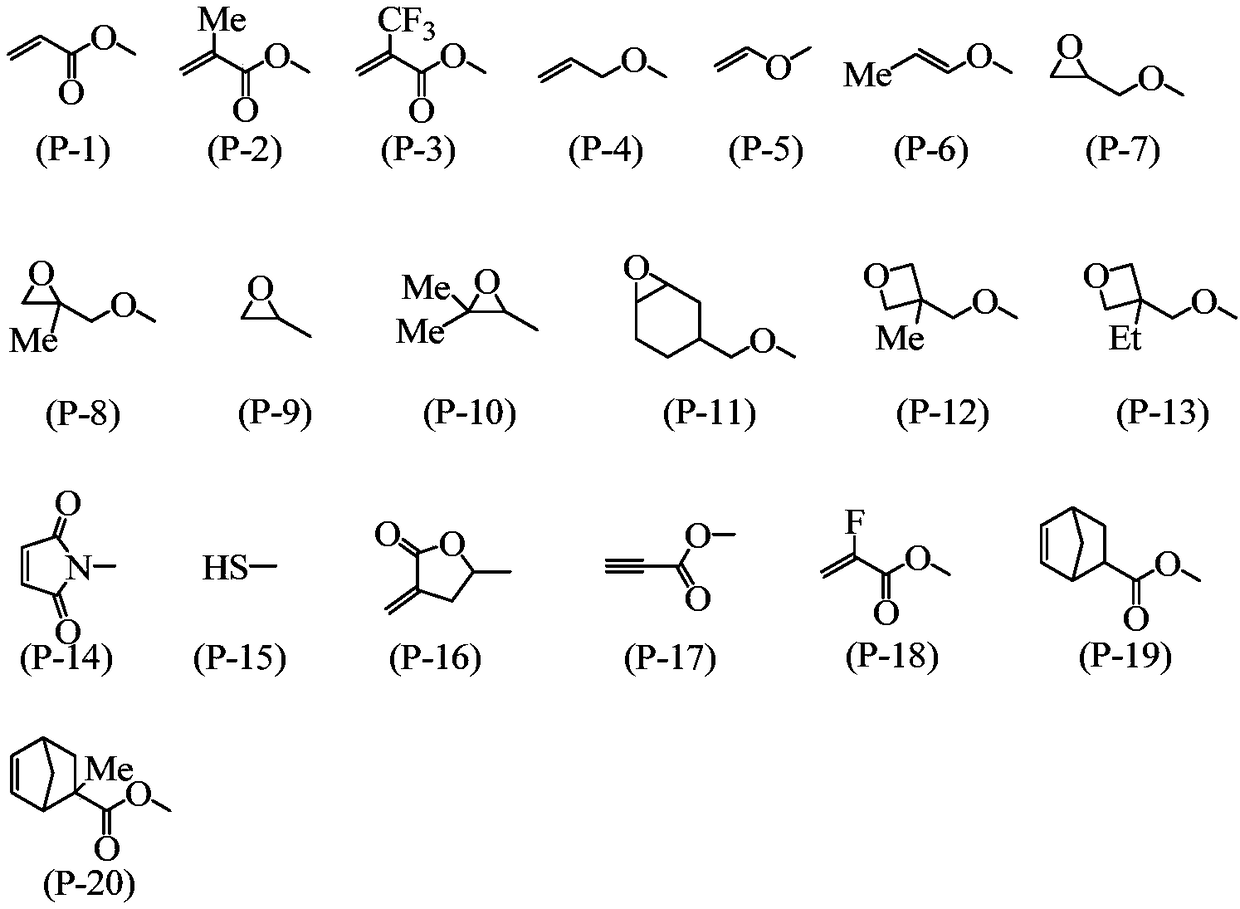
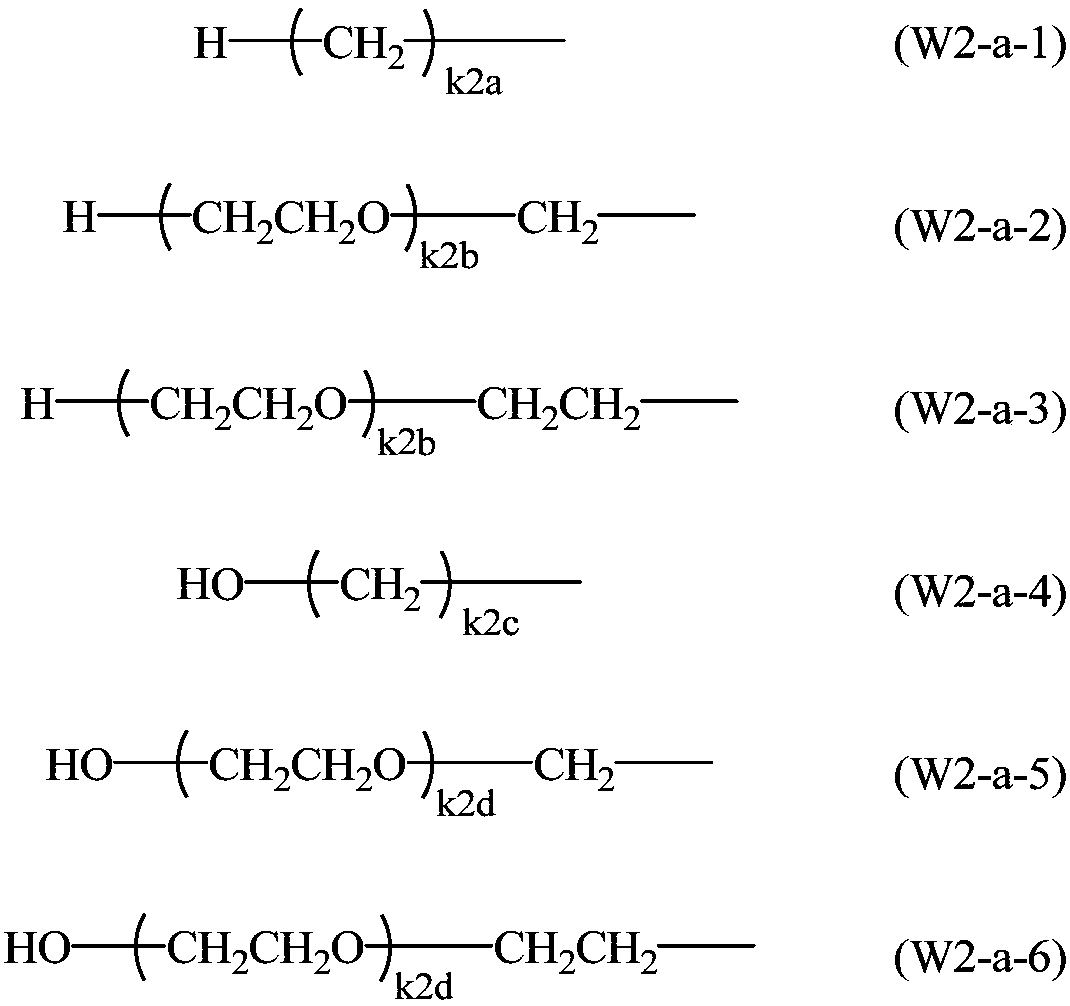Method for producing 2-hydrazinobenzothiazole derivative
A manufacturing method, -CH2- technology, applied to chemical instruments and methods, coatings, instruments, etc., can solve the problem that the separation yield of the target object is not necessarily sufficient, and achieve the reduction of difficult orientation, not easy to change color, and ensure transparency sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] (Example 1) Production of a compound represented by formula (C-1)
[0142] [chem 26]
[0143]
[0144] Under a nitrogen atmosphere, 2.00 g of the compound represented by the formula (C-1-1) and 20 mL of tetrahydrofuran were added to the reaction container. 1.49 g of potassium tert-butoxide was added under ice cooling, and stirred for 2 hours. A solution obtained by dissolving 2.82 g of the compound represented by the formula (C-1-2) in 3 mL of tetrahydrofuran was added dropwise. After stirring at room temperature for 5 hours, it was diluted with 100 mL of dichloromethane, and poured into water. The organic layer was washed with brine, dried over sodium sulfate, and the solvent was distilled off. It purified by column chromatography (silica gel, hexane / ethyl acetate (75:25)), and obtained 2.35 g of the compound represented by Formula (C-1). The yield from the compound represented by formula (C-1-1) was 78%. The reaction solution after the reaction exhibited light...
Embodiment 2
[0149] (Example 2) Production of a compound represented by formula (C-2)
[0150] [chem 28]
[0151]
[0152] Under a nitrogen atmosphere, 2.00 g of the compound represented by the formula (C-2-1) and 20 mL of tetrahydrofuran were added to the reaction container. 0.52 g of sodium amide was added under ice cooling, and the mixture was stirred at room temperature for 2 hours. A solution obtained by dissolving 2.45 g of the compound represented by the formula (C-2-2) in 5 mL of tetrahydrofuran was added dropwise. After stirring at room temperature for 5 hours, it was diluted with 100 mL of dichloromethane, and poured into water. The organic layer was washed with brine, dried over sodium sulfate, and the solvent was distilled off. Column chromatography (silica gel, dichloromethane) and recrystallization (dichloromethane / hexane) were performed to obtain 2.49 g of the compound represented by the formula (C-2). The yield from the compound represented by formula (C-2-1) was 93%...
Embodiment 3
[0157] (Example 3) Production of a compound represented by formula (C-3)
[0158] [chem 30]
[0159]
[0160] Under a nitrogen atmosphere, 2.00 g of the compound represented by the formula (C-3-1) and 20 mL of tetrahydrofuran were added to the reaction container. 0.69 g of sodium methoxide was added under ice cooling, and the mixture was stirred at room temperature for 2 hours. A solution obtained by dissolving 3.17 g of the compound represented by the formula (C-3-2) in 5 mL of tetrahydrofuran was added dropwise. After stirring at room temperature for 5 hours, it was diluted with 100 mL of dichloromethane, and poured into water. The organic layer was washed with brine, dried over sodium sulfate, and the solvent was distilled off. Column chromatography (silica gel, dichloromethane) and recrystallization (dichloromethane / hexane) gave 2.67 g of the compound represented by the formula (C-3). The yield from the compound represented by formula (C-3-1) was 80%. The reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com