Pyridazinone compound and synthesis method thereof
A synthetic method and technology of pyridazinone, which is applied in the field of organic chemical synthesis, can solve problems such as application limitations, and achieve the effects of wide application range, good application prospects, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
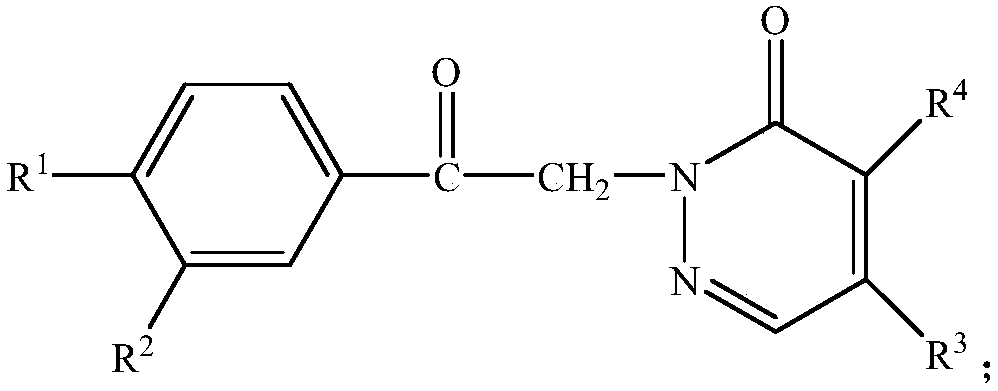
[0036] One pyridazinone compound, the compound molecular formula is as follows:
[0037]
[0038] Among them, R 1 is methyl, R 2 is methyl, R 3 is piperidinyl; R 4 for chlorine. The synthetic method of this pyridazinone compound comprises the following steps:
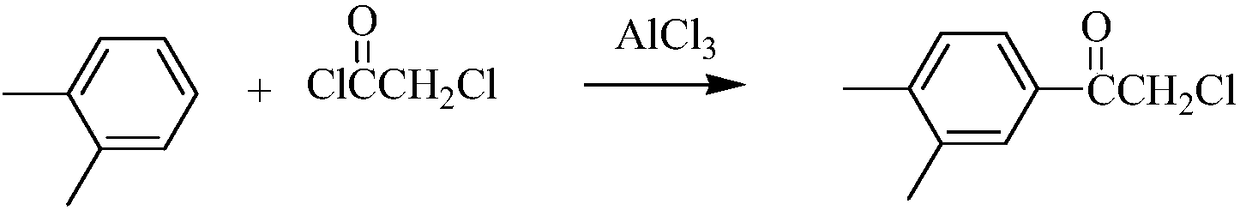
[0039] (1) Synthesis of α-chloroacetophenone
[0040]
[0041] Add 200mL of o-xylene and 74.40g (0.557mol) of anhydrous aluminum chloride into a 250mL three-necked flask with a drying device, cool in an ice-water bath, and then slowly add 60.0g (0.531mol) of α-chloride Substituting acetyl chloride, after the dropwise addition was completed, react at room temperature for 3 hours, and stop stirring. The reaction solution was poured into a beaker filled with 200 mL of ice water and stirred, extracted with ethyl acetate and separated, the organic phase was washed with water until neutral, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to obtain 76.17 g of a white soli...
Embodiment 2
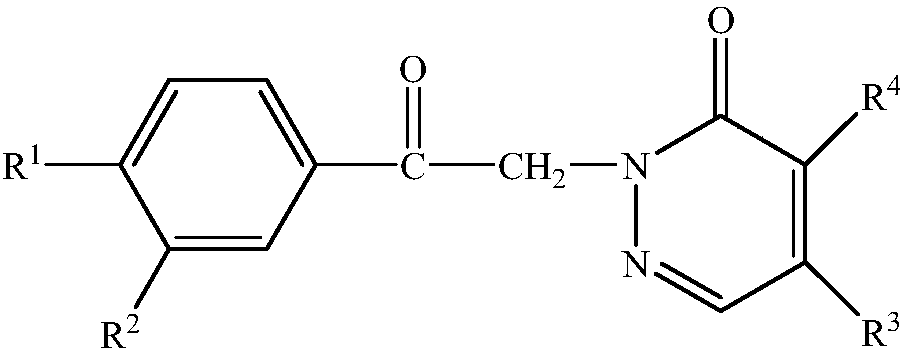
[0052] One pyridazinone compound, the compound molecular formula is as follows:
[0053]
[0054] Among them, R 1 is methyl, R 2 is methyl, R 3 for morpholine, R 4 for chlorine. The synthetic method of this pyridazinone compound comprises the following steps:
[0055] (1) Synthesis of α-chloroacetophenone
[0056]
[0057] Add 200mL of o-xylene and 74.40g (0.557mol) of anhydrous aluminum chloride into a 250mL three-necked flask with a drying device, cool in an ice-water bath, and then slowly add 60.0g (0.531mol) of α-chloride Substituting acetyl chloride, after the dropwise addition was completed, react at room temperature for 3 hours, and stop stirring. The reaction solution was poured into a beaker filled with 200 mL of ice water and stirred, extracted with ethyl acetate and separated, the organic phase was washed with water until neutral, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to obtain 76.17 g of a white soli...
Embodiment 3
[0065] One pyridazinone compound, the compound molecular formula is as follows:
[0066]
[0067] Among them, R 1 is methyl, R 2 is methyl, R 3 is n-propylamino, R 4 for chlorine. The synthetic method of this pyridazinone compound comprises the following steps:
[0068] (1) Synthesis of α-chloroacetophenone
[0069]
[0070] Add 200mL of o-xylene and 74.40g (0.557mol) of anhydrous aluminum chloride into a 250mL three-necked flask with a drying device, cool in an ice-water bath, and then slowly add 60.0g (0.531mol) of α-chloride Substituting acetyl chloride, after the dropwise addition was completed, react at room temperature for 3 hours, and stop stirring. The reaction solution was poured into a beaker filled with 200 mL of ice water and stirred, extracted with ethyl acetate and separated, the organic phase was washed with water until neutral, and dried over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to obtain 76.17 g of a white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com