Method for preparation of dual-dicarboxylic acid diamine lobaplatin (II) derivative by one-pot method
A bis-dicarboxylic acid and dicarboxylic acid technology, which is applied in the field of one-pot preparation of supramolecular platinum-based anti-tumor drug bicycloplatinum, can solve the problems of increased purification difficulty, impossibility of subsequent reaction, and influence, and achieve stable product quality , short production cycle and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
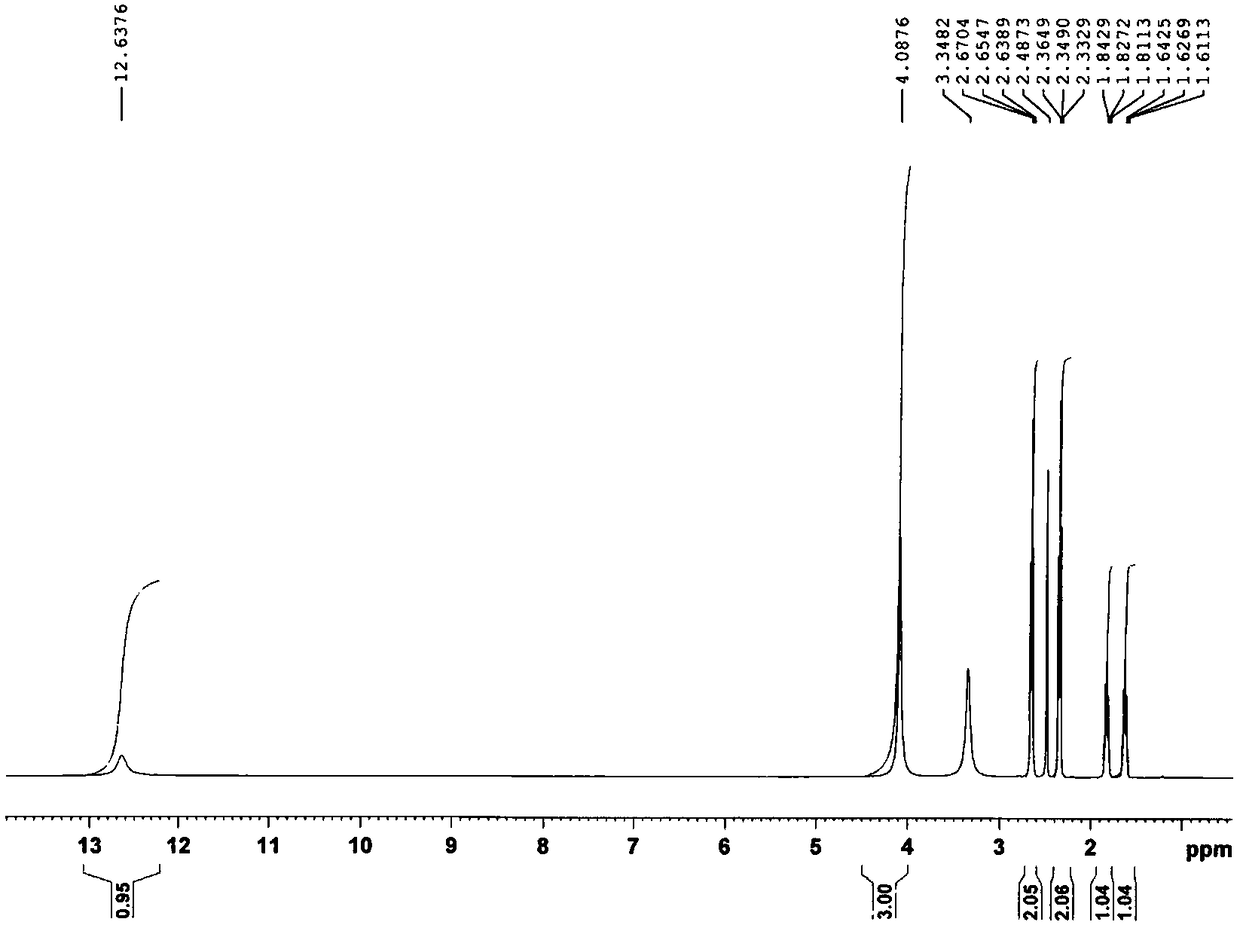
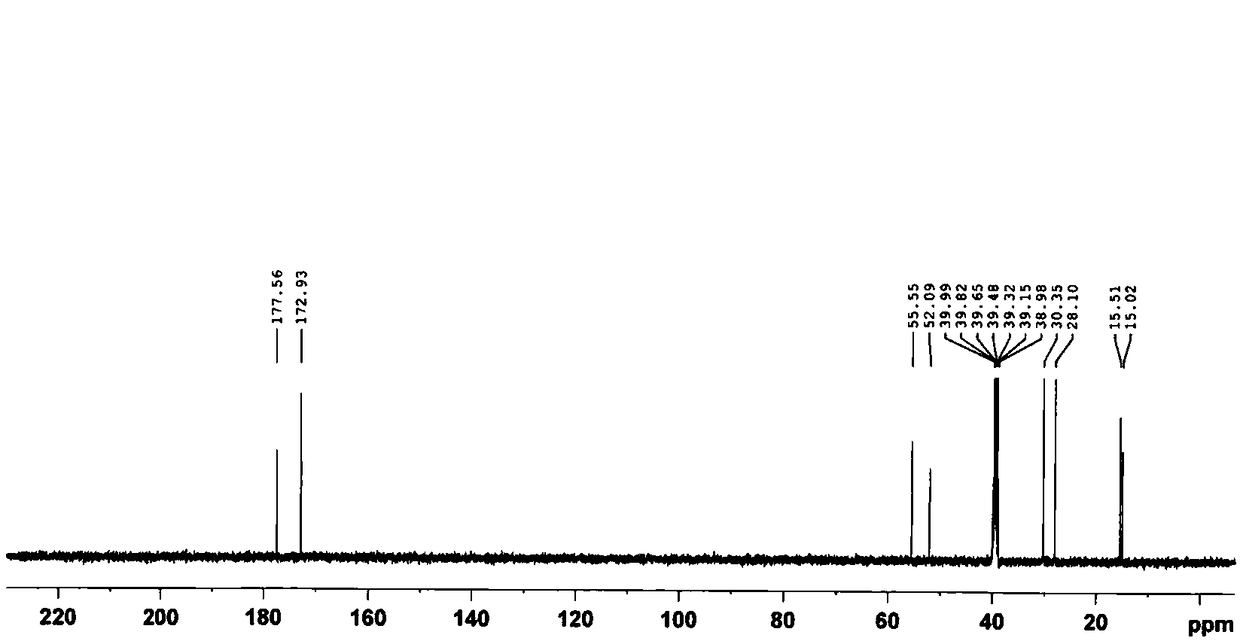
[0121] Take 10.0 g (20.7 mmol) of cis-diiododiammine platinum (II), add 350 ml of purified water, stir evenly and heat to 80°C in a water bath, then add 7.2 g of silver 1,1-cyclobutanedicarboxylate ( 20.1 mmol), reacted for 30 minutes, cooled to 16°C and filtered off the AgI residue, and heated the filtrate to 70°C in a water bath. Add 32.0 g (222.2 mmol) of 1,1-cyclobutanedicarboxylic acid, stir rapidly for 30 minutes, then concentrate under reduced pressure to about 70 ml of remaining liquid, cool to 10°C, filter the precipitated solid, and dry at 60°C to obtain the product 5.43 grams, yield 50.94%, content 100.18%. The obtained product was characterized by elemental analysis, negative ion electrospray mass spectrometry, nuclear magnetic resonance-hydrogen spectrum, nuclear magnetic resonance-carbon spectrum, and X-ray diffraction, and the content of bicycloplatinum was measured by high performance liquid chromatography.
[0122] The result is as follows:
[0123] 1. Eleme...
Embodiment 2
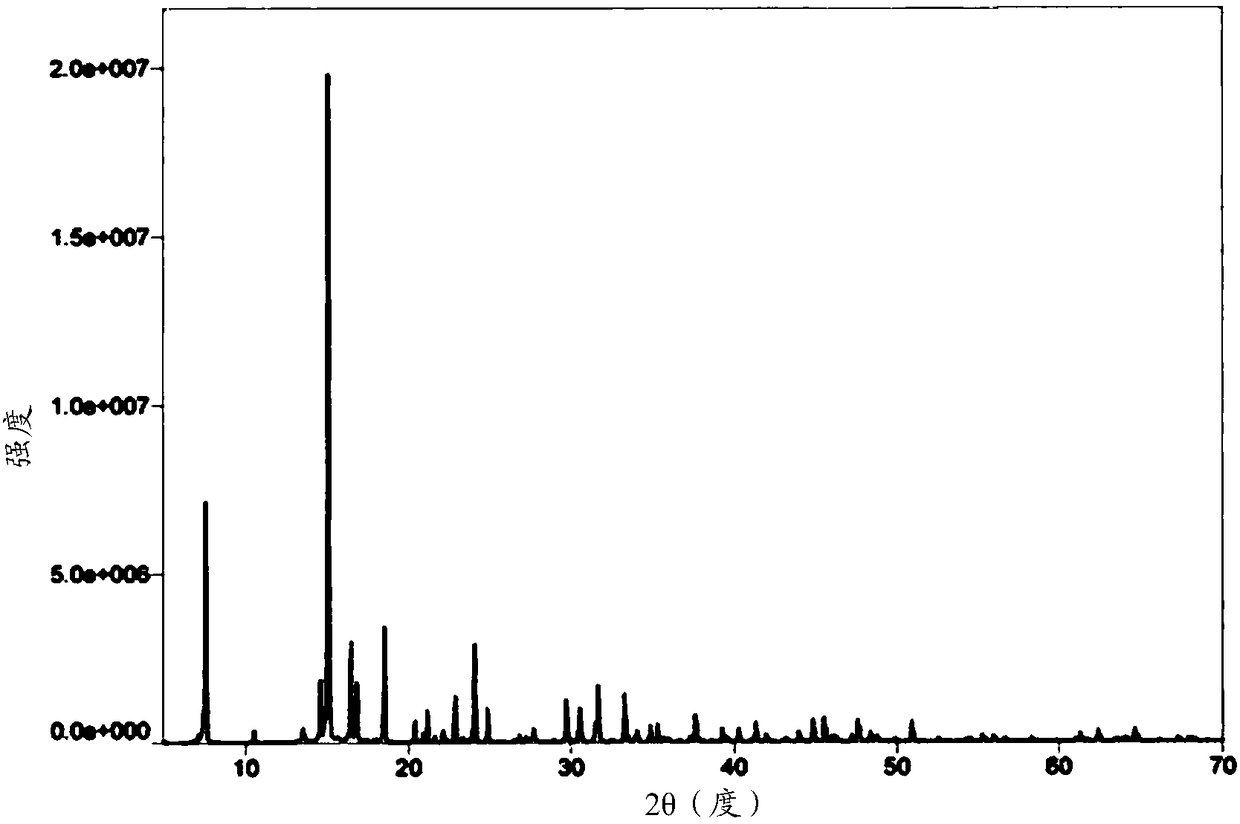
[0142] Example 2 was prepared according to the same method as Example 1, except that 2.98 g (20.7 mmol) of 1,1-cyclobutanedicarboxylic acid was added. As a result, 4.42 g of bicycloplatinum was obtained, with a yield of 41.46% and a content of 101.93%. Negative ion electrospray mass spectrum: 514 (M-1); X-ray diffraction patterns such as Figure 4 shown.
Embodiment 3
[0144] Example 3 was prepared in the same manner as in Example 1, except that 59.6 g (413.9 mmol) of 1,1-cyclobutanedicarboxylic acid was added. As a result, 5.38 g of bicycloplatinum was obtained, with a yield of 50.47% and a content of 98.06%. Negative ion electrospray mass spectrum: 514 (M-1); X-ray diffraction patterns such as Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com