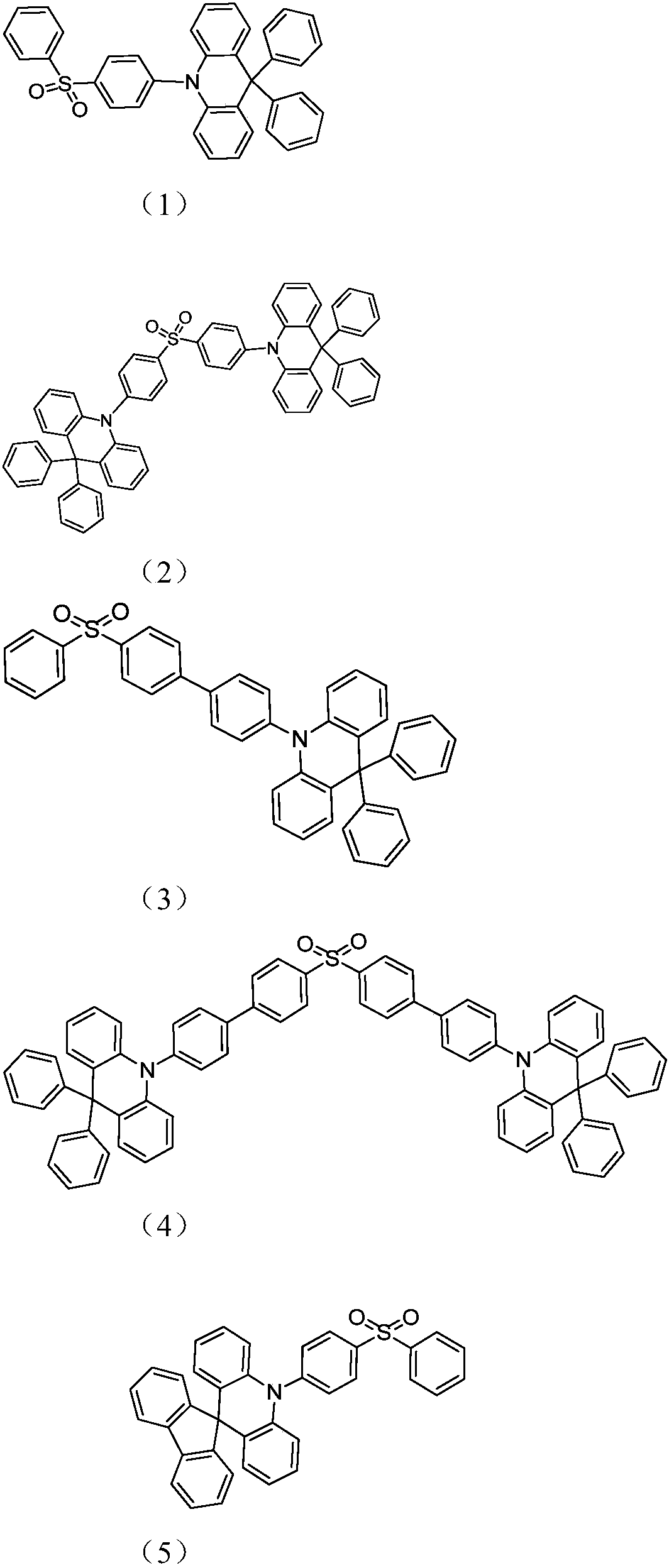
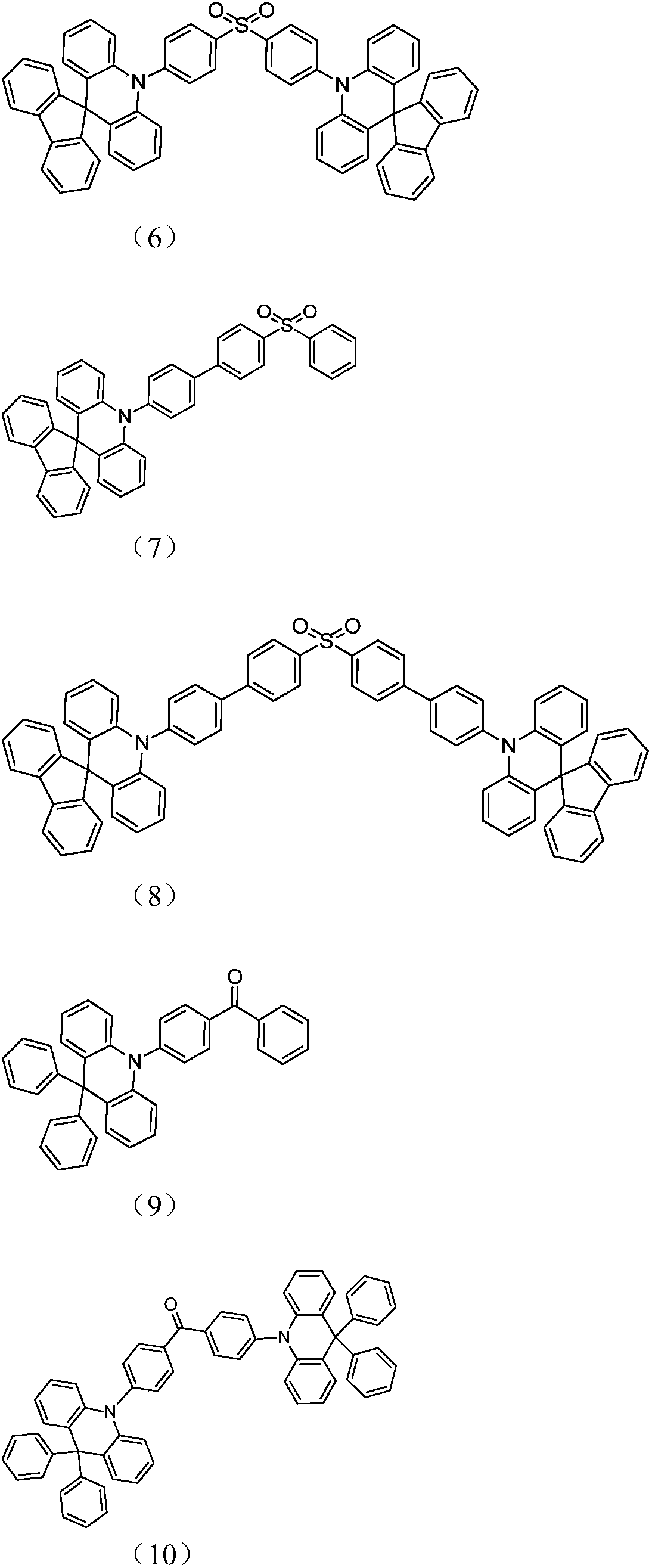
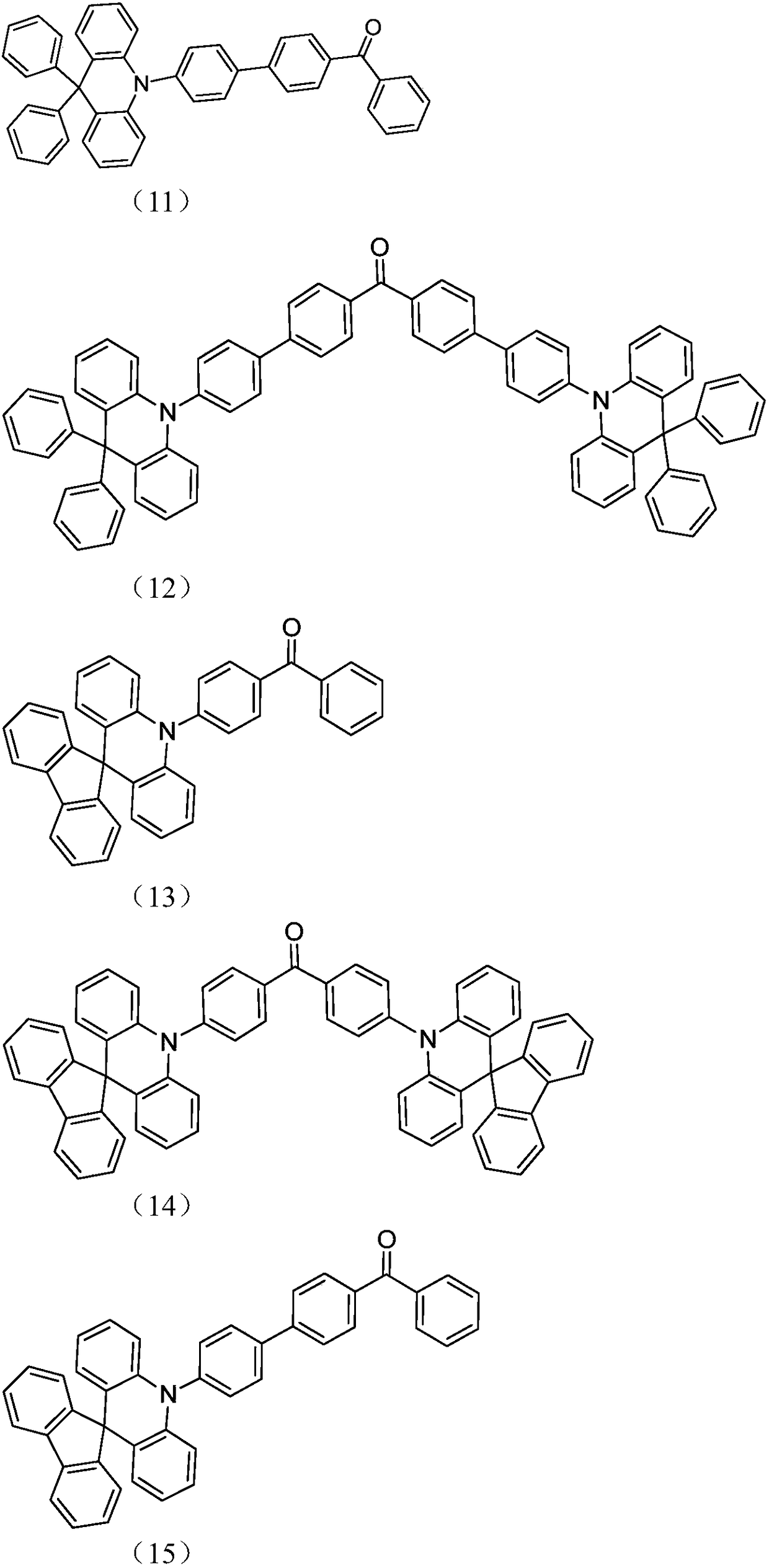
Acridine D-A type thermal activation delayed fluorescent material as well as preparation method and application thereof
A technology of heat-activated delayed and fluorescent materials, which is applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., to achieve the effects of high yield, novel material structure and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In this embodiment, the structural formula of the thermally activated delayed fluorescent material BP-Ph-DPAC is as follows:
[0033]
[0034] The synthetic route of the above-mentioned thermally activated delayed fluorescent material is as follows:
[0035]
[0036] The specific synthesis process is as follows:
[0037] 4-benzoyl-4-bromobiphenyl (0.337g, 1.0mmol), 9,10-dihydro-9,9-diphenylacridine (0.37g, 1.1mmol), tri-tert-butylphosphine tetra Fluoroborate (0.02g) and sodium tert-butoxide (0.2g) were added to a 100ml three-neck flask, vacuum replaced three times with nitrogen, and 20ml of dried toluene was added. Stir at room temperature for 15 min, add Pd 2 (dba) 3 (0.02g), stirred at 120°C for 18h. Cool to room temperature, pass through the elution column (eluted with DCM), spin dry, add silica gel powder for sample preparation, and perform column chromatography separation. 0.54 g of white solid (Y=92%) was obtained, which was recrystallized from hexane / c...
Embodiment 2
[0040] In this embodiment, the structural formula of the thermally activated delayed fluorescent material DPAC-PS-DPAC is as follows:
[0041]
[0042] The synthetic route of the above-mentioned thermally activated delayed fluorescent material is as follows:
[0043]
[0044] The specific synthesis process is as follows:
[0045] 4,4'-dibromodiphenylsulfone (0.11g, 0.3mmol), 9,10-dihydro-9,9-diphenylacridine (0.23g, 0.66mmol), tri-tert-butylphosphine tetrafluoro Add borate (0.02g) and sodium tert-butoxide (0.2g) into a 100ml three-necked flask, vacuumize and replace nitrogen three times, and add 20ml of dried toluene. Stir at room temperature for 15 min, add Pd 2 (dba) 3 (0.02g), stirred at 120°C for 18h. Cool to room temperature, elute through the column (eluted with DCM), spin dry, add silica gel powder to prepare a sample, and perform column chromatography to obtain 0.21 g of white solid (Y=79%).
[0046] NMR analysis product: 1H NMR (400MHz, CDCl3) δ [ppm] 8.05...
Embodiment 3
[0048] In this embodiment, the structural formula of the thermally activated delayed fluorescent material BP-DPAC is as follows:
[0049]
[0050] The synthetic route of the above-mentioned thermally activated delayed fluorescent material is as follows:
[0051]
[0052] The specific synthesis process is as follows:
[0053] Synthesis of BP-DPAC. 4-bromobenzophenone (0.055g, 0.21mmol), 9,10-dihydro-9,9-diphenylacridine (0.077g, 0.23mmol), tri-tert-butylphosphine tetrafluoroborate (0.02g), sodium tert-butoxide (0.2g) was added into a 100ml three-necked flask, vacuum replaced nitrogen three times, and 15ml of dried toluene was added. Stir at room temperature for 15min, add Pd 2 (dba) 3 (0.02g), stirred at 120°C for 18h. Cool to room temperature, pass through the elution column (eluted with DCM), spin dry, add silica gel powder to prepare a sample, and perform column chromatography separation. Reprecipitation with methanol gave 0.09 g of white solid (Y=83.5%).
[005...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com