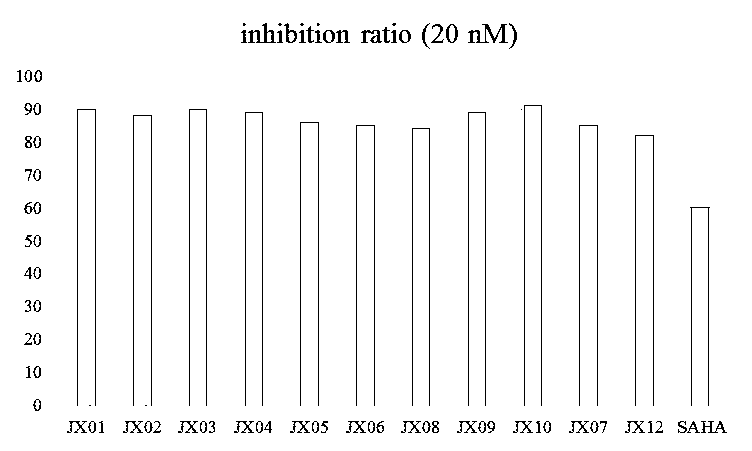
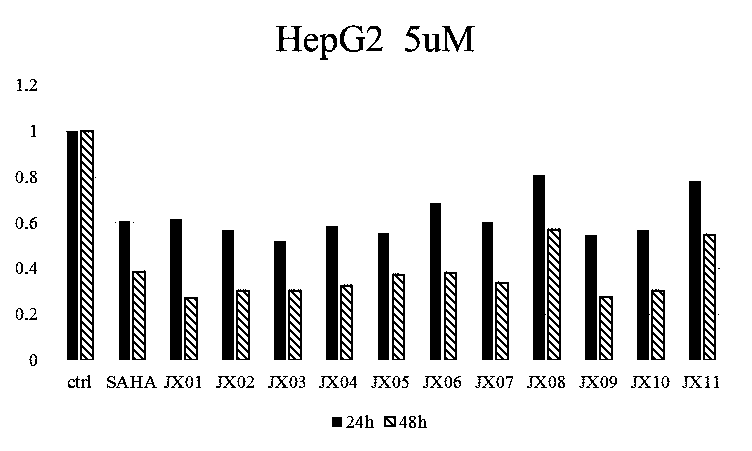
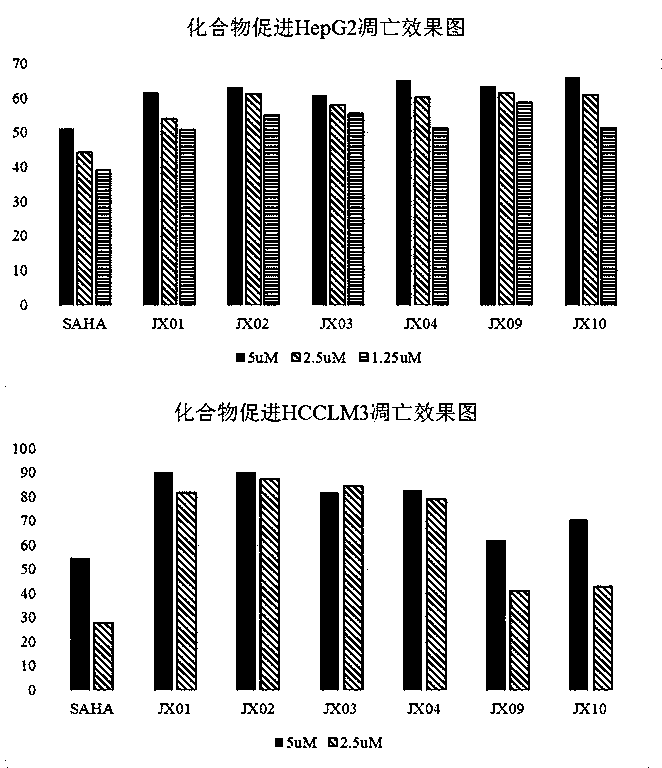
Nitrogen-atom double-substitution hydroxamic acid compound with oxadiazole structure as well as application and preparation method thereof
A technology of oxadiazoles and compounds, which is applied in the field of synthesis of nitrogen-atom double-substituted hydroxamic acid compounds, can solve the problem of unsatisfactory treatment effects on solid tumors, and achieve the effect of promoting acetylation levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0043] Example 1-1, compound N 1 -Hydroxy-N 7 -(4-Methoxyphenyl)-N 7 Preparation of -((5-phenyl-1,2,4-oxadiazole-3-)methyl)pimelic amide (JX01)
[0044] Dissolve p-methylaniline (3.6 g, 30.0 mmol) in DMF, add potassium carbonate (4.1 g, 30.0 mmol), stir for half an hour, then add bromoacetonitrile (1.4 ml, 20.0 mmol), react at room temperature overnight, extract, overnight Column purification gave solid compound 2 (4 g).
[0045] Dissolve compound 2 (3.3 g, 20.0 mmol) in dioxane, add pimelic anhydride (4.3 g, 30.0 mmol), heat to reflux, neutralize acid and alkali after the reaction, evaporate to dryness to obtain compound 3, and directly carry out In the next step of esterification reaction, compound 4 (3.8 g) was purified.
[0046] Dissolve compound 4 (3.8 g, 12.0 mmol) and hydroxylamine hydrochloride (1.25 g, 17.9 mmol) in methanol / water, add sodium carbonate (954 mg, 9.0 mmol), reflux overnight, extract, and purify through a column to obtain a solid compound 5 (2.35 g,...
Embodiment 1-2
[0049] Embodiment 1-2, compound N 1 -Hydroxy-N 7 -(4-Methoxyphenyl)-N 7 Preparation of -((5-(o-fluorophenyl)-1,2,4-oxadiazole-3-)methyl)pimelic acid amide (JX02)
[0050] Replace benzoyl chloride with o-fluorobenzoyl chloride, and prepare JX02 according to the method for preparing compound JX01. 1 H NMR (600MHz, DMSO) δ 10.29 (brs, 1H), 8.64 (brs, 1H), 8.02 (dd, J = 7.2, 7.8 Hz, 1H),7.67-7.66 (m, 1H), 7.48 – 7.43 (m, 1H), 7.42 – 7.36 (m, 2H), 7.33 (dd, J =8.4, 9.0 Hz, 1H), 7.03 (d, J = 8.4 Hz, 1H), 6.98 (d, J = 7.8 Hz, 1H), 5.76(s, 2H), 3.78 (s, 3H), 2.07 (t, J = 7.8 Hz, 2H), 1.87-1.85 (m, 2H), 1.45-1.43(m, 2H), 1.39 – 1.36 (m, 2H), 1.14 – 1.10 (m, 2H).
Embodiment 1-3
[0051] Embodiment 1-3, compound N 1 -Hydroxy-N 7 -(4-Methoxyphenyl)-N 7 Preparation of -((5-(m-fluorophenyl)-1,2,4-oxadiazole-3-)methyl)pimelic acid amide (JX03)
[0052] Benzoyl chloride was replaced by m-fluorobenzoyl chloride, and JX03 was correspondingly prepared according to the method for preparing compound JX01. 1 H NMR (600MHz, DMSO) δ 10.31 (brs, 1H), 8.65 (brs, 1H), 7.96 (d, J = 7.8 Hz, 1H), 7.89(d, J = 9.0, 1H), 7.72-7.68 (m, 1H), 7.59 (td, J = 8.4, 2.4 Hz, 1H), 7.33 (d, J = 9.0 Hz, 2H), 6.99 (d, J = 9.0 Hz, 2H), 4.99 (s, 2H), 3.77 (s, 3H), 2.04(t, J = 7.2 Hz, 2H), 1.88 (t, J = 7.2 Hz, 2H), 1.48 – 1.42 (m, 2H), 1.41 –1.35 (m, 2H), 1.16 – 1.10 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com