Atom transfer free radical polymerization method regulated and controlled by inorganic metal salt
An inorganic metal salt and atom transfer technology, which is applied in the field of new and efficient controlled free radical polymerization, can solve the problems of difficult storage and handling, unstable properties of iodohydrocarbons, limiting the scope of application of the polymerization system, etc. The effect of reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
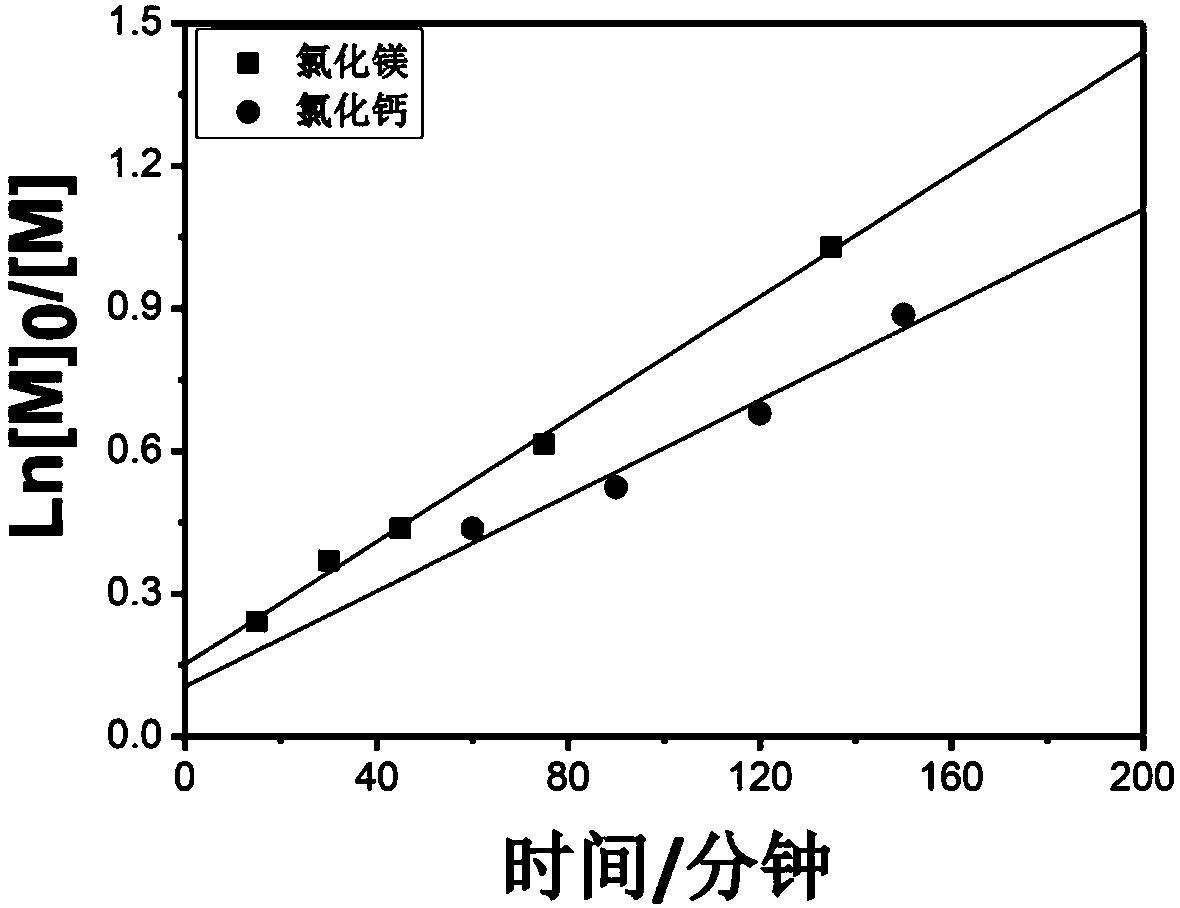
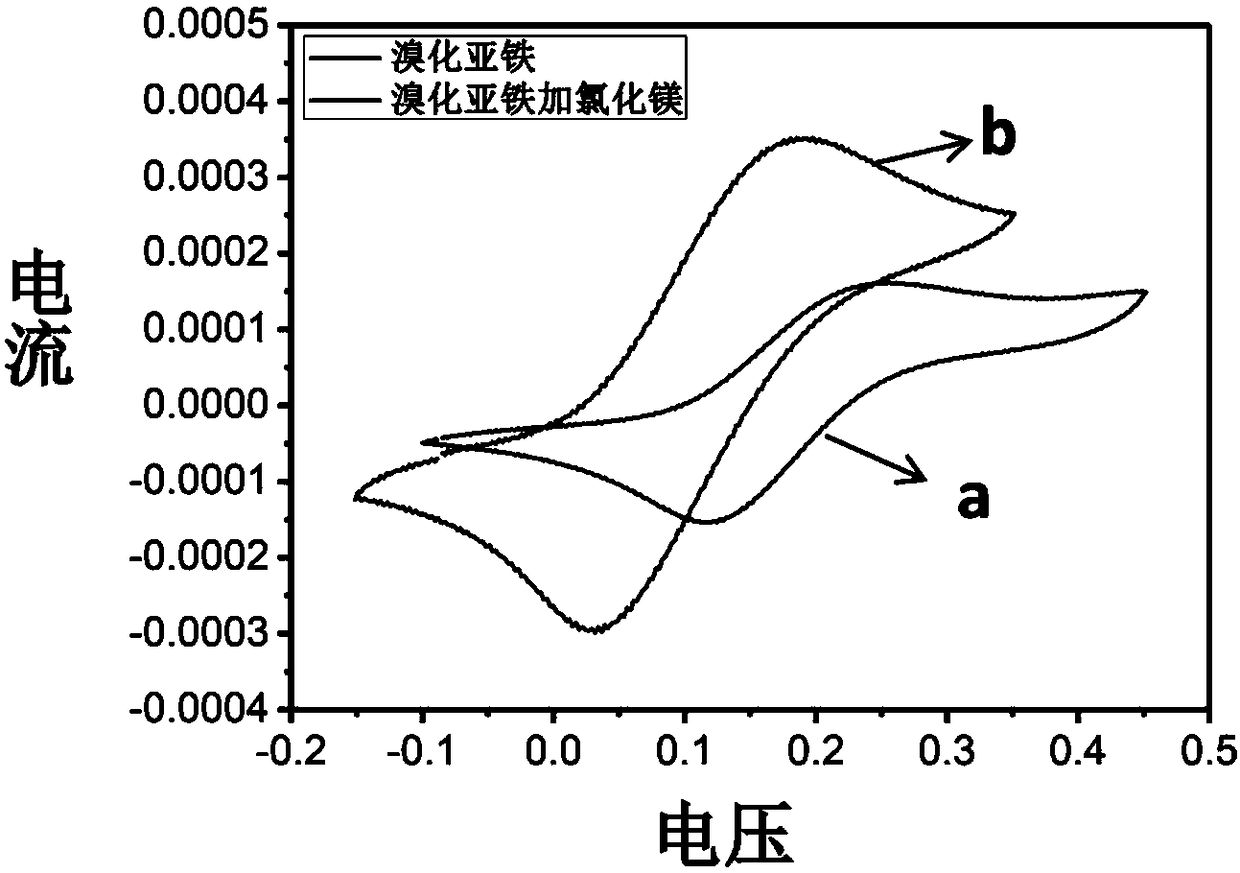
[0042] After the methyl methacrylate monomer, ethyl 2-bromoisobutyrate initiator and magnesium chloride are purified, the polymerization reaction components are prepared, methyl methacrylate, ethyl 2-bromoisobutyrate, ferrous bromide The molar ratio to magnesium chloride is 200:1:1:2. The preparation process is as follows: in the glove box, 0.061 gram of ferrous bromide and 0.054 gram of magnesium chloride are weighed and put into an eggplant-shaped bottle, and 6 milliliters of methyl methacrylate monomer is added into the eggplant-shaped bottle, and after magnetic stirring for 5 minutes, Then 37.7 microliters of ethyl 2-bromoisobutyrate was added, and stirring was continued for 2 minutes to obtain a pre-reaction mixture.
[0043] Transfer the pre-reaction mixture in the eggplant-shaped bottle to the heating device, and control the reaction temperature to 70°C; after the polymerization reaction reaches the predetermined reaction time interval, take a certain amount of the mixt...
Embodiment 2
[0045] After purification of methyl methacrylate monomer, ethyl 2-bromophenylacetate, halogenated hydrocarbons, and inorganic metal salts, the preparation of polymerization reaction components, methyl methacrylate, ethyl 2-bromophenylacetate, bromine The molar ratio of ferrous chloride and inorganic metal salt is 200:1:1:2. The preparation process is as follows: in the glove box, 0.061 grams of ferrous bromide and the corresponding quality of inorganic metal salts are put into an eggplant-shaped bottle, 6 milliliters of methyl methacrylate monomer is added to the eggplant-shaped bottle, and magnetically stirred for 5 Minutes later, 49.5 microliters of ethyl 2-bromophenylacetate was added, and stirring was continued for 2 minutes to obtain a pre-reaction mixture.
[0046] Transfer the pre-reaction mixture in the eggplant-shaped bottle to the heating device, and control the reaction temperature to 70°C; after the polymerization reaction reaches the predetermined reaction time in...
Embodiment 3
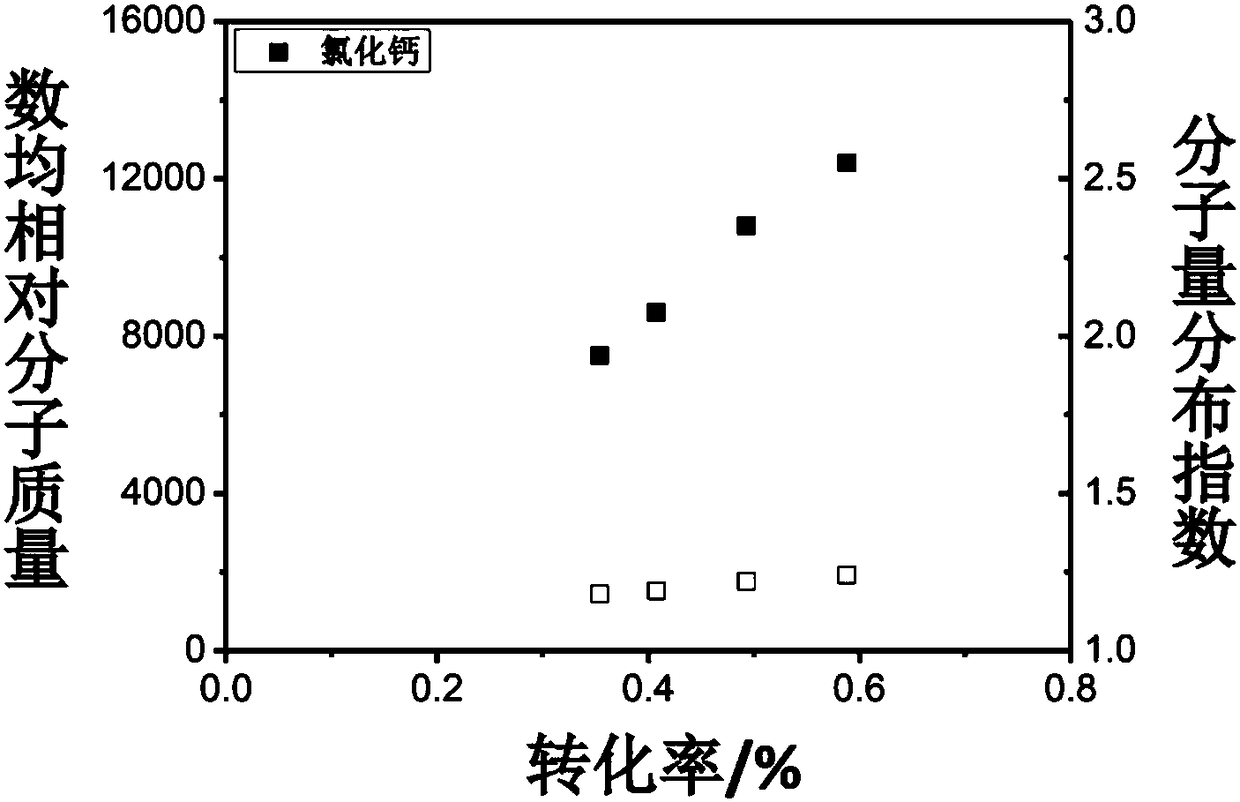
[0049] After purification of methyl methacrylate monomer, ethyl 2-bromophenylacetate halogenated hydrocarbon and calcium chloride, the preparation of polymerization reaction components, methyl methacrylate, ethyl 2-bromophenylacetate, bromine The molar ratio of ferrous chloride and potassium bromide is 200:1:1:4. The preparation process is as follows: weigh 0.061 grams of ferrous bromide and 0.062 grams of calcium chloride in the glove box and put them into an eggplant-shaped bottle, add 6 milliliters of methyl methacrylate monomer into the eggplant-shaped bottle, and stir magnetically for 5 minutes Then, 49.5 microliters of ethyl 2-bromophenylacetate was added, and the stirring was continued for 2 minutes to obtain a pre-reaction mixture.
[0050] Transfer the pre-reaction mixture in the eggplant-shaped bottle to the heating device, and control the reaction temperature to 70°C. After the polymerization reaction reaches the predetermined reaction time interval, take a certain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com