LMP-2 recombinant adeno-associated virus vector and construction method and application thereof
A technology of LMP-2 and viral vectors, applied in the direction of virus/bacteriophage, application, virus, etc., can solve the problems of low virus titer, limited therapeutic gene capacity, rAAV stability damage, etc., and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. Construction and detection of recombinant adeno-associated virus vector rAAV / LMP-2 and rAAV / mLMP-2
[0046] Materials and their sources:
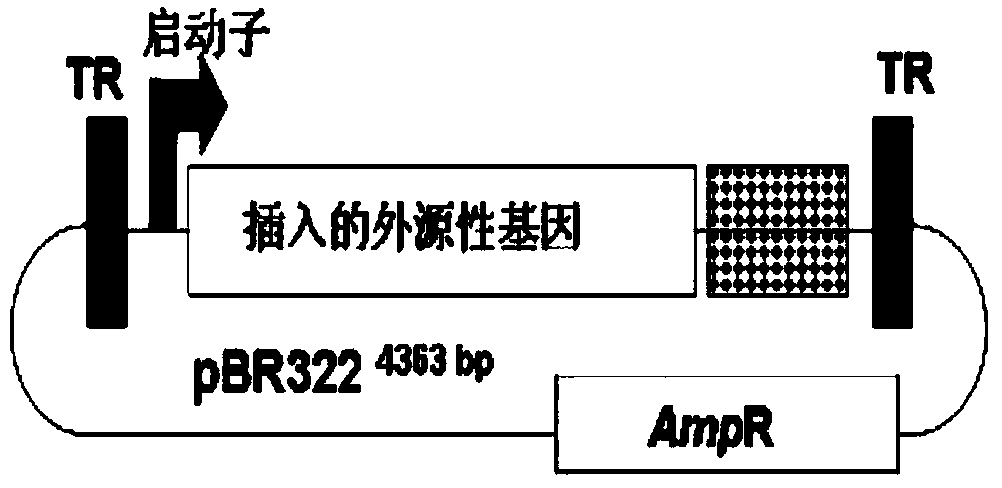
[0047] A. The pBR322 plasmid (named pBR-AAV2) carrying the whole genome DNA of AAV type 2: It was prepared by Professor Paul L. Hermonat, one of the main technical directors of Broadway Gene International Co., Ltd. (Hermonat, P.L., and Muzyczka, N .Use of adeno-associated virus as a mammalian DNA cloning vector:transduction of neomycin resistance into mam malian tissue culture cells.Proc.Natl.Acad.Sci.U.S.A.81:6466-6470.).
[0048] B. Human nasopharyngeal carcinoma cells: isolated from cancer tissues of patients with nasopharyngeal carcinoma, immunohistochemically confirmed LMP-2 positive (or purchased from commercial channels).
[0049] C. The pCI-neo plasmid carrying the CMV promoter was purchased from Promega Corporation in the United States, and the plasmid pSG424 carrying the SV40 early promoter was purchased from ...
Embodiment 2
[0060] Embodiment 2, preparation of recombinant adeno-associated virus (rAAV) and virus titer determination
[0061] Materials and their sources:
[0062] A. The recombinant adeno-associated virus vector rAAV / LMP-2 carrying the LMP-2 gene constructed in Example 1 and the recombinant adeno-associated virus vector rAAV / mLMP-2 carrying the LMP-2 mutant gene (rAAV / AmLMP-2, rAAV / BmLMP-2, rAAV / CmLMP-2 and rAAV / DmLMP-2).
[0063] B. Auxiliary plasmid pHelper containing the Rep gene and Lip / Cap gene of AAV: constructed by Professor Liu Yong from the Gene Therapy Center of the Affiliated Hospital of the University of Arkansas School of Medicine (Liu, Y., Chiriva-Internati, M., Grizzi, F. Salati , E., Roman, J.J., Lim S., and Hermonat, P.L. Rapid induction of cytotoxic T cell response against cervical cancer cells by human papilloma virus type16E6 antigen gene delivery into human dendritic cells by an aden o-associated virus vector. Cancer 8: Gene The 948-957.).
[0064] C. AAV-HEK...
Embodiment 3
[0084] Example 3. Tumor killing experiment of introducing tumor-associated antigen into monocyte-macrophage-dendritic cell line
[0085] Materials and their sources:
[0086] A. rAAV viruses: rAAV / LMP-2 and rAAV / mLMP-2 (rAAV / AmLMP-2, rAAV / BmLMP-2, rAAV / CmLMP-2).
[0087] B. AIM-V cell culture medium: purchased from Invitrogen, USA.
[0088] C. Cytokines: Colony cell stimulating factor (GM-CSF), interleukins 2, 4, 7 (IL-2, 4, 7) and tumor necrosis factor (TNF-α) were purchased from American R&D Corporation.
[0089] 1. Tumor killing experiment
[0090] Such as Figure 5 As shown, the whole process of the tumor-killing experiment based on one or more rAAV viruses carrying tumor-associated antigen genes (LMP-2 gene and mutant gene thereof) of the present invention infecting tumor patient's monocytes includes the following steps:
[0091] A. Take 50-150 ml of peripheral blood from a tumor patient, use a blood cell separator (or lymphocyte separation medium) to obtain periphe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com