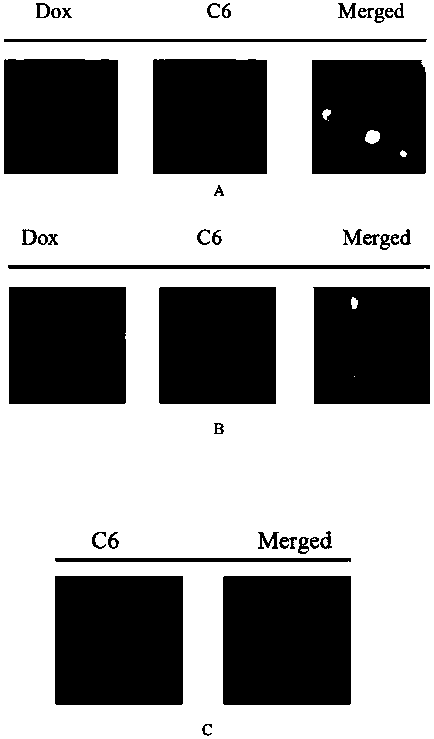
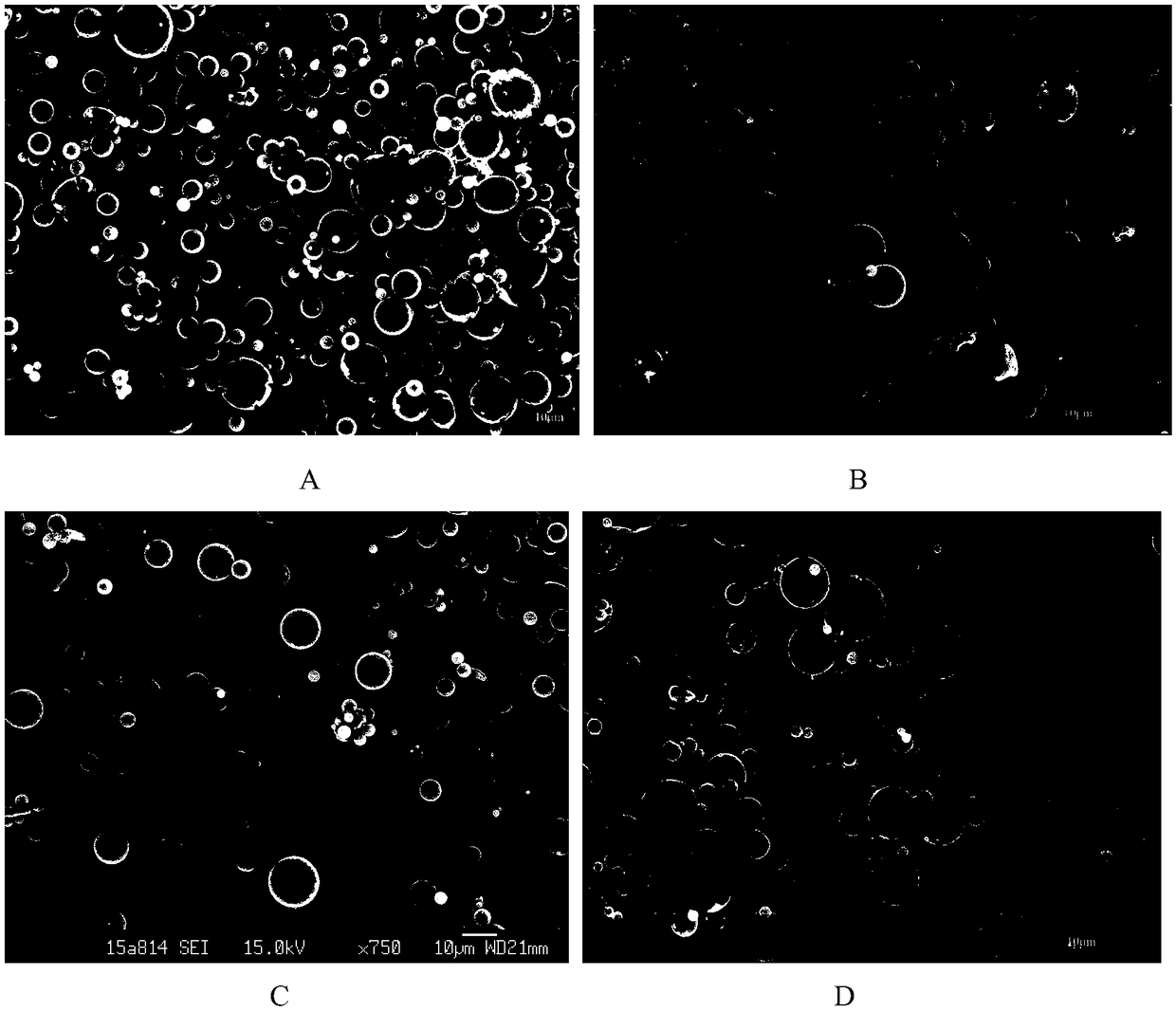
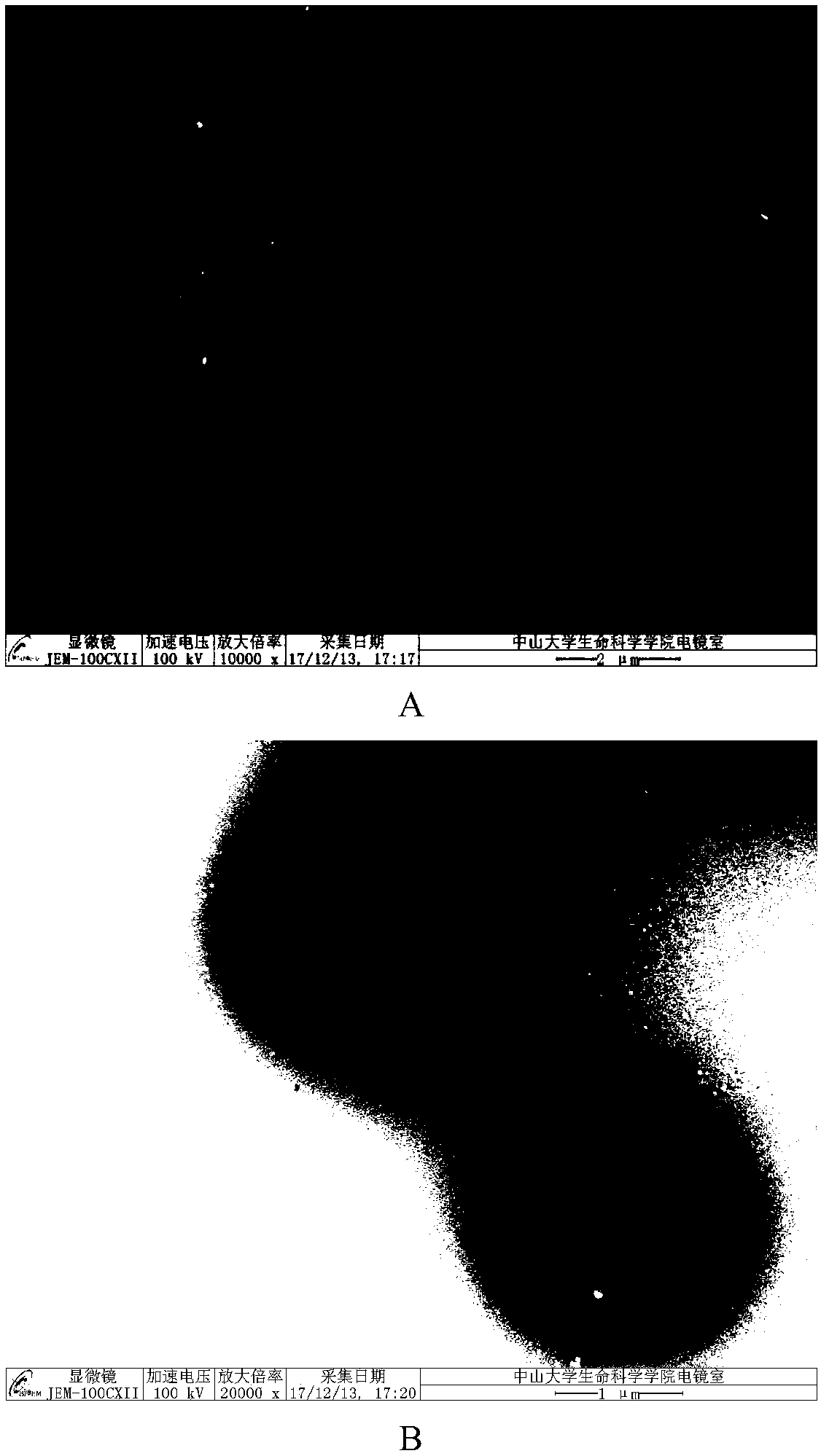
Composite micro-sphere co-carrying adriamycin nanoparticles and ginsenoside rh2 and preparation method thereof
A technology of doxorubicin hydrochloride and ginsenosides, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, microcapsules, etc., can solve the problem of unsatisfactory clinical test results and affect the pharmacokinetics of anticancer drugs It can improve the anti-tumor effect, reduce the toxic and side effects, and achieve the effect of high encapsulation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] This example provides a composite microsphere co-loaded with doxorubicin hydrochloride nanoparticles and ginsenoside rh2. The preparation method is as follows:
[0053] (1) Precisely weigh a certain amount of doxorubicin hydrochloride, and prepare a doxorubicin hydrochloride solution with a concentration of 0.4 mg / ml with ultrapure water, and set aside. Accurately weigh a certain quality of sodium alginate (MW=75KDa) and chitosan (MW=50KDa), and prepare 3mg / ml sodium alginate solution and chitosan solution with ultrapure water, respectively, and adjust the pH to 6.00 and 5.50, filtered through a 0.22 μm filter membrane for later use. Place 10ml of 3mg / ml sodium alginate solution on a stirrer with a stirring speed of 500rpm, add 10ml of 0.4mg / ml doxorubicin hydrochloride solution, then slowly add 20ml of 3mg / ml chitosan solution dropwise to form doxorubicin hydrochloride Mycin polyelectrolyte nanoparticle suspension. The nanoparticle suspension was centrifuged at 15000...
Embodiment 2
[0062] This example provides a composite microsphere co-loaded with doxorubicin hydrochloride nanoparticles and ginsenoside rh2. The preparation method is as follows:
[0063] (1) Precisely weigh a certain amount of doxorubicin hydrochloride, and prepare a doxorubicin hydrochloride solution with a concentration of 0.4 mg / ml with ultrapure water, and set aside. Accurately weigh a certain quality of sodium alginate (MW=75KDa) and chitosan (MW=50KDa), and prepare 3mg / ml sodium alginate solution and chitosan solution with ultrapure water, respectively, and adjust the pH to 6.00 and 5.50, filtered through a 0.22 μm filter membrane for later use. Place 10ml of 3mg / ml sodium alginate solution on a stirrer with a stirring speed of 500rpm, add 10ml of 0.4mg / ml doxorubicin hydrochloride solution, then slowly add 20ml of 3mg / ml chitosan solution dropwise to form doxorubicin hydrochloride Mycin polyelectrolyte nanoparticle suspension. The nanoparticle suspension is centrifuged at 15000r...
Embodiment 3
[0070] This example provides a composite microsphere co-loaded with doxorubicin hydrochloride nanoparticles and ginsenoside rh2. The preparation method is as follows:
[0071] (1) Precisely weigh a certain amount of doxorubicin hydrochloride, and prepare a doxorubicin hydrochloride solution with a concentration of 0.4 mg / ml with ultrapure water, and set aside. Accurately weigh a certain quality of sodium alginate (MW=75KDa) and chitosan (MW=50KDa), and prepare 3mg / ml sodium alginate solution and chitosan solution with ultrapure water, respectively, and adjust the pH to 6.00 and 5.50, filtered through a 0.22 μm filter membrane for later use. Place 10ml of 3mg / ml sodium alginate solution on a stirrer with a stirring speed of 500rpm, add 10ml of 0.4mg / ml doxorubicin hydrochloride solution, then slowly add 20ml of 3mg / ml chitosan solution dropwise to form doxorubicin hydrochloride Mycin polyelectrolyte nanoparticle suspension. The nanoparticle suspension is centrifuged at 15000r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com