Metal organic complex and organic light emitting device thereof
A technology of organic complexes and metals, which is applied in the field of metal-containing organic complexes and organic light-emitting devices, can solve the problems that high-performance red phosphorescent materials need to be further developed, and achieve good thermal stability and chemical stability. properties, reduction of vibration energy loss, and effects of high-efficiency luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
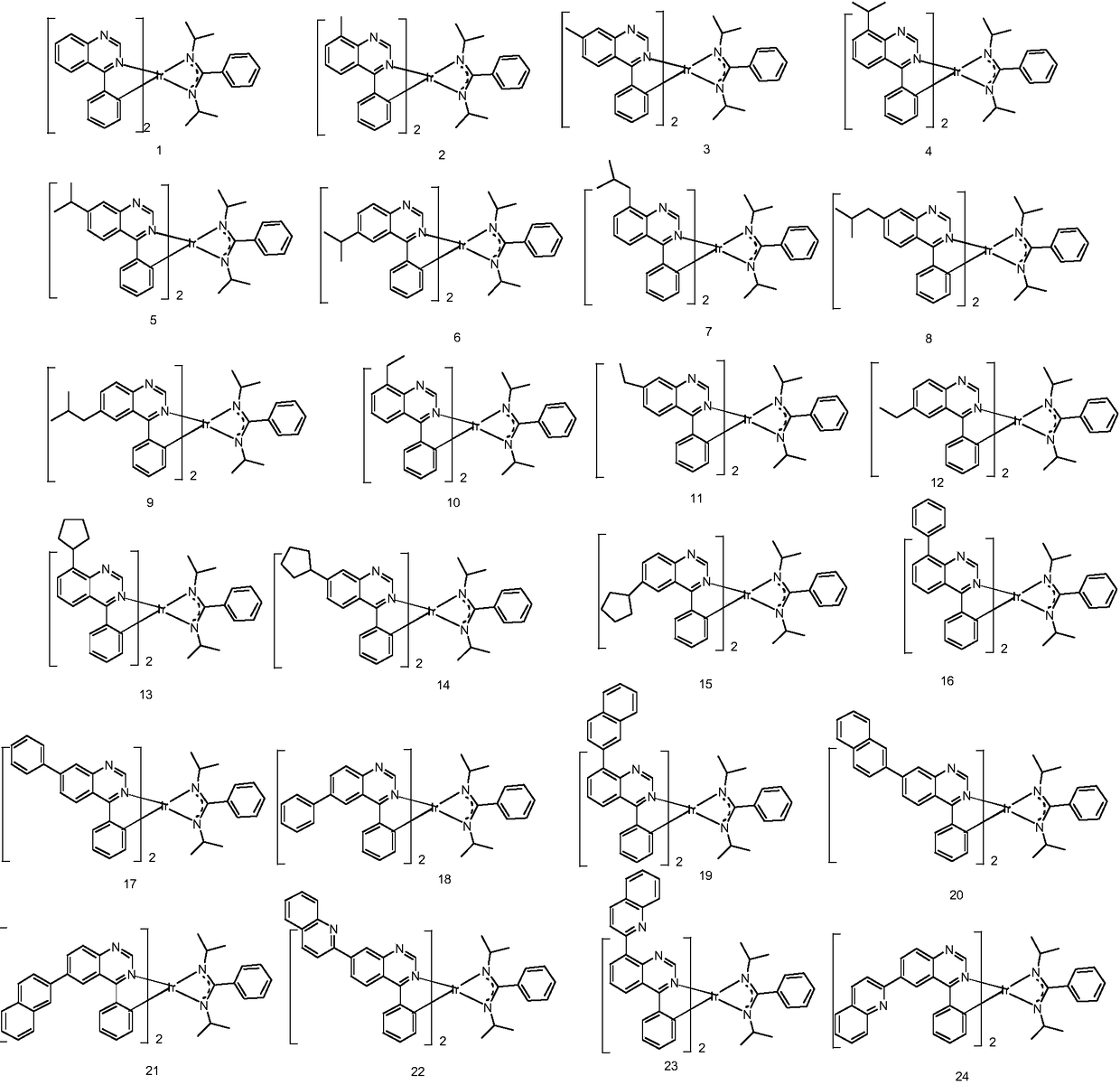
[0104] Embodiment 1: the preparation of compound 1
[0105]
[0106] Preparation of intermediate A1
[0107] Phenylboronic acid (5.3g, 38.4mmol), 4-bromoquinazoline (6.4g, 30.7mmol) and K 2 CO 3 (8.5g, 61.4mmol) was dissolved in toluene (150ml) and water (30ml). The mixture was degassed by bubbling it with nitrogen for 30 minutes, and tetrakistriphenylphosphopalladium Pd (PPh 3 ) 4 (1.8 g, 1.53 mmol). The mixture was then kept at 100°C overnight. After completing the reaction, the mixture was cooled to room temperature, extracted with toluene, and washed with brine and water. The organic solution was filtered and evaporated. The crude product was purified by column chromatography using a gradient mixture of ethyl acetate in heptane (20% to 65%). The white powder product was recrystallized three times from heptane to give colorless crystals c1. In a 1L three-necked flask, iridium trichloride hydrate (14.1g, 40mmol) and compound c1 (35.1g, 170mmol) were added, then 3...
Embodiment 2
[0113] Embodiment 2: the preparation of compound 5
[0114] The b1 in Example 1 was replaced by equimolar b5, and the other steps were the same as in Example 1 to obtain the target compound 5 (5.17 g, 29%).
[0115]
[0116] Mass Spectrum m / z: 891.38 (calculated: 891.37). Theoretical element content (%)C 47 h 50 IrN 6 : C, 63.35; H, 5.66; Ir, 21.57; N, 9.43 The measured element content (%): C, 63.36; H, 5.65; Ir, 21.58; N, 9.42. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0117] Embodiment 3: the preparation of compound 16
[0118] The 4-bromoquinazoline in Example 1 was replaced with equimolar b16, and the other steps were the same as in Example 1 to obtain the target compound 16 (5.56 g, 29%).
[0119]
[0120] Mass Spectrum m / z: 959.35 (calculated: 959.34). Theoretical element content (%)C 53 h 46 IrN 6 : C, 66.37; H, 4.83; Ir, 20.04; N, 8.76 The measured element content (%): C, 66.38; H, 4.82; Ir, 20.04; N, 8.76. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com