Polymer drug carrier with aggregation-induced luminescence and dual sensitivity, drug-loaded micelles and preparation method thereof
An aggregation-induced luminescence and dual-sensitivity technology, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of reduced fluorescence efficiency and achieve improved biological safety and circulation The effect of time improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
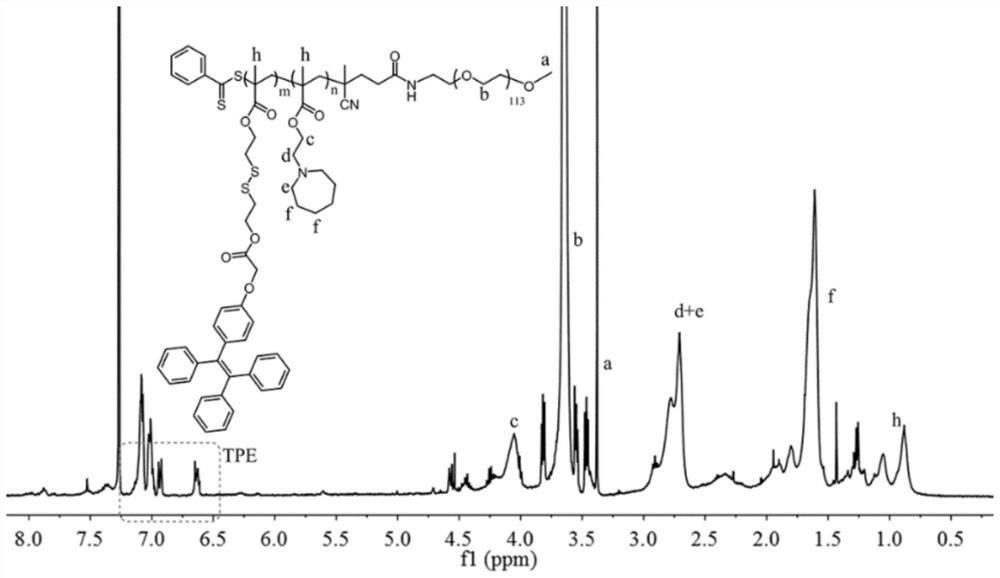
[0039] A polymer drug carrier with aggregation-induced luminescence and dual sensitivity, polymethacrylate block and polyethylene glycol block; wherein, the polymethacrylate block is grafted with disulfide bonded tetraphenylethylene and hydroxyethylhexamethyleneimine.
[0040] The preparation method of the above-mentioned polymer drug carrier with aggregation-induced luminescence and dual sensitivity, the reaction process is as follows:
[0041]
[0042] Specifically include the following steps:
[0043] (1) Under the protection of argon, dissolve tetraphenylethylene (1g) with carboxyl groups and dihydroxyethyl disulfide (0.56g) with methacrylic acid at one end in anhydrous dichloromethane, and then add Dicyclohexylcarbodiimide (0.76g) and 4-dimethylaminopyridine (0.03g), stirred at room temperature for 24h, then purified by column chromatography, and finally dried to obtain tetraphenylethylene methacrylic acid with a disulfide bond ester monomer;
[0044] (2) Dissolve t...
Embodiment 2
[0047] A polymer drug carrier with aggregation-induced luminescence and dual sensitivity, polymethacrylate block and polyethylene glycol block; wherein, the polymethacrylate block is grafted with disulfide bonded tetraphenylethylene and hydroxyethylhexamethyleneimine.
[0048] The preparation method of the above-mentioned polymer drug carrier with aggregation-induced luminescence and dual sensitivity, the reaction process is as follows:
[0049]
[0050] Specifically include the following steps:
[0051] (1) under argon protection, tetraphenylethylene (0.5g) with carboxyl and dihydroxyethyl disulfide (0.5g) that one end is connected with methacrylic acid are dissolved in anhydrous dichloromethane, then Add dicyclohexylcarbodiimide (0.5g) and 4-dimethylaminopyridine (0.02g), stir at room temperature for 24h, then purify by column chromatography, and finally dry to obtain tetraphenylethylenemethyl with disulfide bond Acrylate monomer;
[0052] (2) Dissolve the monomer (0.3...
Embodiment 3
[0055] A polymer drug carrier with aggregation-induced luminescence and dual sensitivity, polymethacrylate block and polyethylene glycol block; wherein, the polymethacrylate block is grafted with disulfide bonded tetraphenylethylene and hydroxyethylhexamethyleneimine.
[0056] The preparation method of the above-mentioned polymer drug carrier with aggregation-induced luminescence and dual sensitivity, the reaction process is as follows:
[0057]
[0058] Specifically include the following steps:
[0059] (1) under argon protection, tetraphenylethylene (1.5g) with carboxyl and dihydroxyethyl disulfide (1.5g) that one end is connected with methacrylic acid are dissolved in anhydrous dichloromethane, then Add dicyclohexylcarbodiimide (1.8g) and 4-dimethylaminopyridine (0.04g), stir at room temperature for 24h, then purify by column chromatography, and finally dry to obtain tetraphenylethylenemethyl with disulfide bond Acrylate monomer;
[0060] (2) Dissolve the monomer (0.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
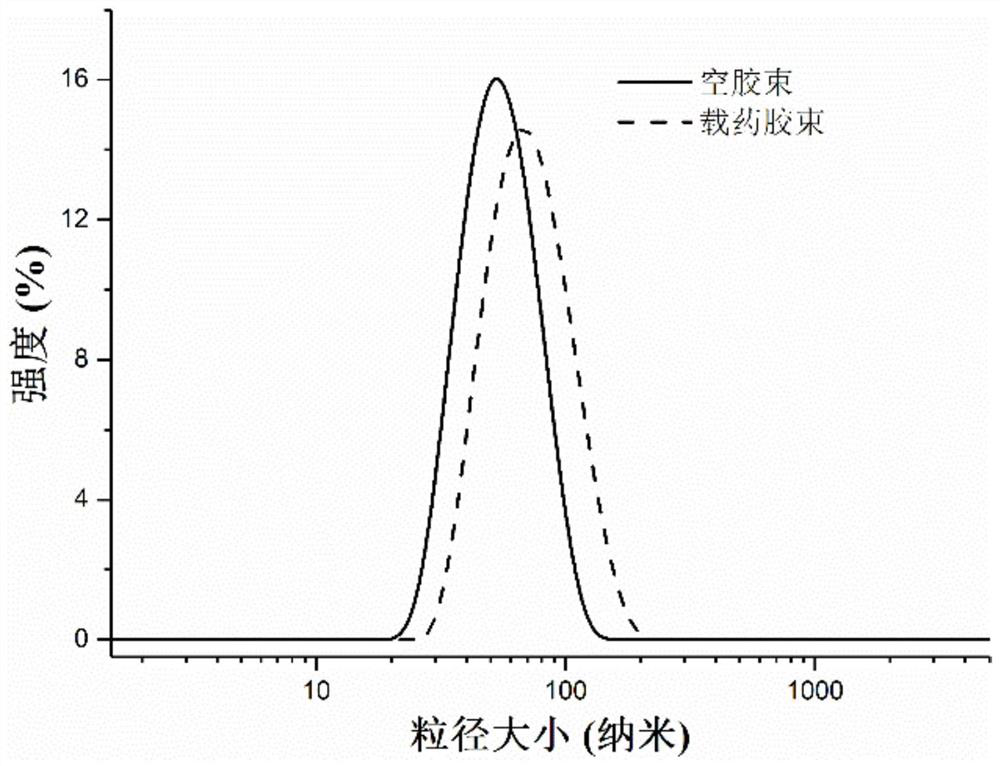
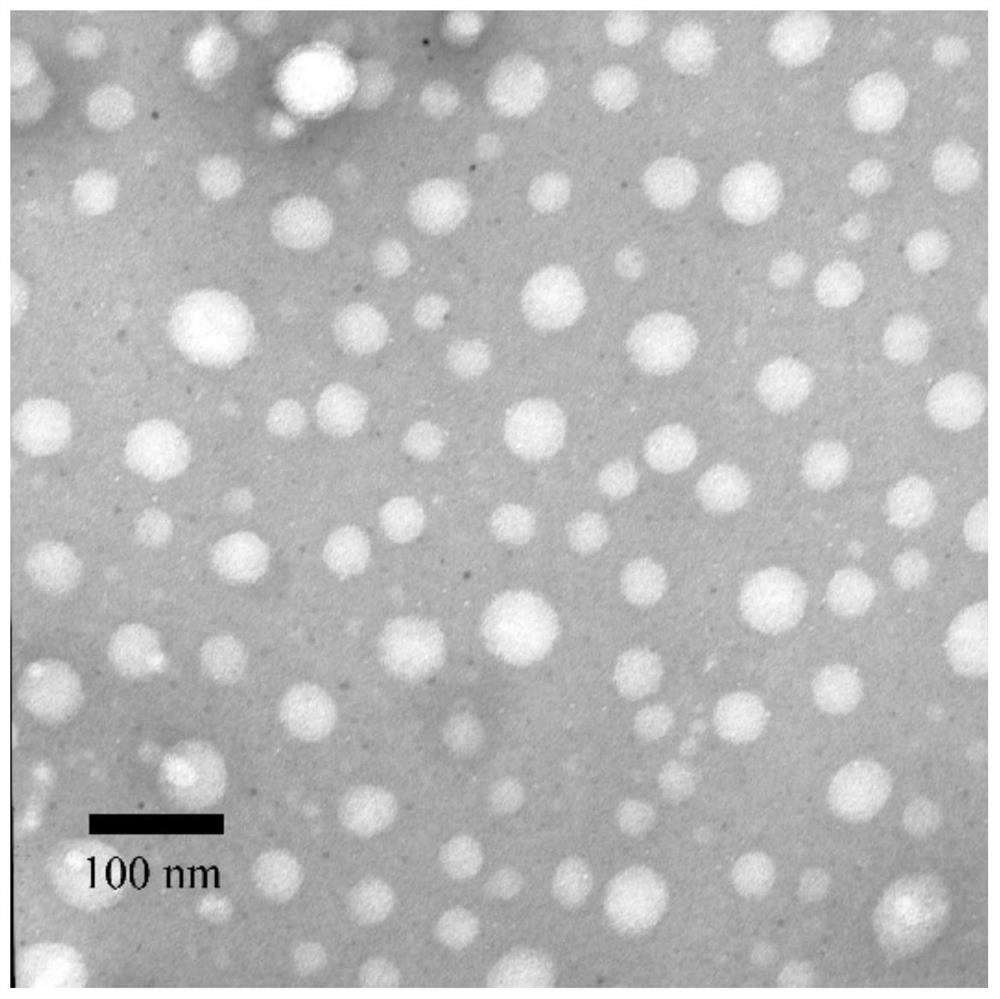
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com